The Importance of Knowing the Ct Value at which COVID PCR Tests are Positive
ABSTRACT/SUMMARY:
Throughout the COVID-19 pandemic the SARS-CoV-2 PCR test (hereafter referred to as the COVID PCR test) has played the major role in diagnosis of individual patients and collection of epidemiological data.
To date, this test has been reported as being either positive or negative, with no indication on the report as to whether the result was strongly positive or only weakly positive. This has made it difficult for people and their physicians to know whether, at one extreme, a person is infected with a huge load of active “live” contagious virus that greatly threatens the person and those around them; or, at the other extreme, the test has detected only a trace amount of inert, non-infectious, “dead” viral debris that does not pose a threat to the person or to others, and might even represent a false positive result.
Not knowing where a person fits along the above spectrum hampers optimal care of patients with severe COVID illness and creates unnecessary fears regarding people at the other end of the spectrum.
Most people who die from COVID (the disease caused by the SARS-CoV-2 virus) probably die primarily because their immune system greatly over-reacts to the virus—resulting in a harmful, life-threatening hyperinflammatory state, often with “cytokine storm.” Most likely, only a minority of people who die from COVID do so because of overwhelming viral infection. In some patients with severe illness both problems are present (hyperinflammation and overwhelming viral infection); in other patients only one of these two problems is present—usually hyperinflammation.
Life-threatening hyperinflammation requires prompt and aggressive lifesaving immunosuppression, while overwhelming viral infection requires inhibition of viral replication (anti-viral therapies). Since it can be dangerous to give immunosuppressive treatment to patients who are carrying a large viral load, it is desirable to know, at least roughly, what a patient’s viral load is when administration of needed immunosuppression is contemplated.
Fortunately, the PCR test can be used to estimate viral load, as well as degree of contagiousness. These estimates can be gleaned from the Ct value at which the test is positive. Though these estimates are not perfect, they can be helpful.
Ct = Cycle threshold; Ct = the number of amplification cycles needed before the test detects presence of viral material in a specimen. The Ct value is the inverse of the viral load. The higher the Ct needed to detect the viral material, the lower the viral load and the less sick and contagious the person is likely to be. If a test is positive at a Ct of 12 (becomes positive after only 12 amplification cycles), the viral load might be 100,000,000 copies per microliter, or more. If the test becomes positive only at a Ct of 37, 40, or 45, the result most likely represents either a false positive, or a true positive that is detecting a trace amount (less than 100 copies) of inert, non-contagious, “dead” SARS-CoV-2 viral debris.
Knowing the Ct value at which a severely ill patient’s COVID test is positive, would be immensely helpful to a physician who would like to know how much of a viral load the patient is carrying and whether it is relatively safe (or not) to administer life-saving immunosuppression, if careful monitoring reveals need for the latter. By using serial Ct values for guidance, the precision and timing of treatment of severe COVID illness can be markedly improved. This, in turn, might reduce morbidity, mortality, need for mechanical ventilation, duration of hospital and ICU stays, and cost of care.
Awareness of Ct values is also essential for optimal interpretation of, and reaction to, positive COVID tests that result from surveillance screening. For example, if an asymptomatic person with no definite COVID exposure is found to have a positive test at a Ct of 40, the test is most likely detecting only trace amounts of inert, non-infectious viral material, or is a false positive. In either case, it is likely that the person is not personally in danger, does not represent a danger to others, and does not need to be quarantined.
It is one thing if mass surveillance testing reveals 100,000 positive COVID tests and all 100,000 are positive at a Ct <30; it is quite a different matter if 90,000 of the 100,000 tests are positive only at a Ct of 40.
Inclusion of Ct information in all epidemiologic studies would greatly improve the accuracy, scientific quality, and meaningfulness of data collected, regarding new and cumulative COVID cases, COVID hospitalizations, and COVID deaths—and, in turn, this would markedly improve the quality of public education, public understanding, public dialogue, and public policy decisions—all of which are fundamentally based on these data.
To date, there has been an insufficient amount of true, constructive dialogue about COVID. Instead, there has been a dangerous amount of polarization, extremism, over-simplification, confusion, and intolerance of different views. Perhaps widespread public awareness and discussion of Ct values could provide clarity, bring people in from the extremes, and facilitate respectful, collaborative problem-solving.
Bottom Line: Morbidity, mortality, hospital and ICU crowding, health care worker shortage, health care costs, individual and public angst, public fears, public polarization, public confusion, and public policy mistakes could all be markedly decreased by understanding and optimally paying attention to the Ct values at which COVID tests are positive.
UNDERSTANDING THE VALUE AND LIMITATIONS OF THE COVID PCR TEST:
On 11/10/20 CNN reported that during the preceding week [1]:
- 119,238 “new cases of COVID” were occurring per day (on average)
- “Soon, there will likely be 200,000 new cases occurring per day.”
- 59,000 “new COVID hospitalizations” were occurring per day.
- More than 1000 “new COVID deaths” were occurring per day.
- 1.5 million COVID tests were being conducted per day.
The quality and meaningfulness of the above data depend, fundamentally, on the quality, reliability, wise use, and wise interpretation of the COVID PCR tests upon which these data are based.
The COVID PCR test: The PCR test for COVID-19 is an excellent test when used appropriately and interpreted wisely. It is designed to detect presence of 1-3 small, unique segments (small RNA sequences) of the known SARS-CoV-2 virus genome. It does not test for presence of the entire genome; just a small portion(s) of the genome. It is specific, at least when manufactured properly and used by an excellent lab to assess a person who has (or has had) clinical features suggestive of active COVID infection.
Ct values and viral load: PCR testing is extremely sensitive. [2-9] Thanks to amplification techniques, the PCR test for COVID (or any virus) can detect trace amounts of viral material. The amount of virus, i.e., the “viral load,” in a patient’s nasopharynx can be grossly estimated by noting how many times the PCR amplification process needs to be repeated to produce enough viral genetic material for the test to detect. In other words, the viral load is reflected by the number of amplification cycles needed to detect presence of the viral material. That number of amplification cycles is called the Cycle threshold (Ct). [2, 3, 8, 9]
If the viral load is very high, fewer amplification cycles are needed to detect presence of viral material—i.e., virus is detected at a low Ct. If only trace amounts of viral material are present, many amplification cycles (a high Ct) will be needed to detect that tiny amount of viral material. The higher the Ct needed to detect the viral material, the lower the viral load and the less sick and contagious the person is likely to be. [2, 3, 8, 9]
So, Ct = Cycle threshold, and Ct = the number of amplification cycles needed before the test can detect the viral material. The Ct value is the inverse of the viral load. [2, 3, 8, 9]
If a person’s PCR test detects presence of virus at a Ct of 8 (i.e., if the test becomes positive after only 8 amplification cycles), the viral load may be in the range of billions of viral RNA copies per microliter. [8] A positive test at a Ct of 12 correlates with at least 100,000,000 copies. [8] If a person’s test becomes positive only at a Ct of 37, 40, or 45, this typically corresponds to presence of less than 100 copies, even as little as 1 copy per microliter. [2, 3, 8, 9]
Most commercial labs are using a Ct of 37 or 40 to detect presence of COVID. In other words, if no viral material is detected after 30 or 35 amplification cycles, but at least a tiny amount of genetic material (even 1 copy per microliter, as opposed to 100 million copies) is detected after 37 or 40 cycles (at Ct of 37 or 40), that person’s test is declared “positive.” [8] Officials at the WHO have recommended a Ct cutoff of 45 (i.e., the test is declared positive even if it only becomes positive after 45 amplification cycles).
Specificity: For the COVID test to be specific for COVID, it must detect a portion of the SARS-CoV-2 virus genome that is unique to the SARS-CoV-2 virus and not found in any other virus. Manufacturers of COVID PCR tests must be careful to ensure that their test does not detect genome fragments that, yes, are present in the SARS-CoV-2 virus, but are also present in other viruses, including other coronaviruses.
More than 150 for-profit COVID PCR tests have been rushed onto the market [10], and most of these have not been adequately vetted by the FDA or any other independent entity. They have been granted “emergency use authorization (EUA),” but have otherwise not gone through the usual more rigorous official FDA approval process. Rushed production and marketing of these test kits has been lucrative business.
It is likely that these tests are, as claimed by their manufacturers, highly specific for the SARS-CoV-2 virus [4, 5, 11-16] and do not cross-react with genetic material of common coronavirus [4, 6, 7], or other genetic material—at least when they are used in idealized situations to test patients with quite active COVID (e.g., patients whose clinical features are compellingly compatible with COVID and whose COVID PCR test is positive at a Ct less than 30).
Most of the test manufacturers state that their test is 99-100% specific for detection of the SARS-CoV-2 virus and never cross-reacts with other coronaviruses or any other viruses. Most publications have stated a specificity in the range of 95-100%, most commonly in the 98.1-99.8 range. [4, 5, 11-16] This would mean an expected false positivity rate of no more than 5% and usually between 0-2%. One publication, however, has mentioned a specificity of only 92%, which would mean a false positivity rate of 8%. [4]
It will be important to eventually ask independent, unbiased quality control commissions to rigorously verify the specificity of these tests—not just when used in patients whose clinical characteristics are compelling for a diagnosis of COVID (diagnostic testing), but particularly when used to screen people who are asymptomatic or have mild and non-specific symptoms (surveillance testing).
False positivity: False positive COVID PCR test results have been proven to occur [11, 12, 17-22], most likely due to: contamination during sampling, contamination by PCR amplicons, contamination of reagents, sample cross-contamination, and, conceivably, cross-reactions with non- SARS-CoV-2 virus genetic material. For example, the USA CDC had to withdraw its initial COVID PCR testing kits (in March 2020), because they were shown to have a high rate of false-positives due to reagent contamination. [23]
The exact incidence of false-positivity is still unclear, but preliminary independent studies, based on meta-analysis of RNA PCR tests in general, as well as COVID PCR tests, suggest that the false positivity rate is most likely somewhere between 0·8% and 4·0%, probably less than 1%—at least when excellently manufactured tests are used in experienced labs to evaluate patients with clinical features that are quite compatible with COVID. [11, 12]
The likelihood of a positive test result being a “false positive” increases as the pretest probability of COVID infection decreases. Furthermore, weakly positive results, due to trace amounts of viral material, amounts that are close to the assay’s limits of detection (LOD), have a greater likelihood of being a false positive than is the case with samples that are loaded with huge quantities of virus. When an asymptomatic person who has had no compelling reason to think they have COVID is tested as part of a screening process and has a positive test at a high Ct (37 or greater), the likelihood of this result being a false positive is greater than is the case of a similar result in someone who has (or has had) clinical features strongly suggestive of COVID.
It will be important, therefore, to determine the incidence of false positivity across the entire Ct spectrum and across the entire spectrum of illness (from never symptomatic to severely ill). It is likely that the incidence of false positivity is extremely low in patients with floridly active COVID infection—i.e., in patients with classical and severe COVID symptoms and a positive COVID test at a Ct in the teens or lower 20s, or at least less than 31. However, it is likely that the incidence of false positivity is much higher (possibly higher than 4%?) in people whose COVID test is positive only at a Ct of 37 or 40, particularly if they are part of a screening process and have been asymptomatic or minimally and non-specifically symptomatic. [12, 17, 18]
There has been an unfortunate dearth of adequate studies of the incidence of false positivity in the surveillance setting. Furthermore, the false positivity rate may vary greatly from one test manufacturer to another, and from one lab to another.
What does a positive result at a Ct of 37 or higher mean? In an Italian study [17], a total of 1639 nasopharyngeal swabs (NPS) were analyzed for COVID by PCR— using an Xpert® Xpress SARS-CoV-2 (Cepheid) PCR test. Of the 1639 swabs, 36 (2.3%) were positive; the rest were negative. Seventeen of the 36 positive patients (47%) were positive only at a high Ct. [For example, in 14 patients only the N2 gene was detected, with Ct between 38.0 and 43.4, while in 3 samples the E and N genome regions were both amplified, with high Ct (37.6-45).]
To confirm positivity, the above-mentioned 17 samples were retested with Cepheid and two other PCR methods (Altona or Elitech). Five of the 17 (29%) appeared to be true positives. However, 12 of the 17 (70.58%) proved to be false positives. Only 2 of these 12 people were hospitalized patients with COVID-19-like symptoms; the rest had not been suspected to have COVID and had been screened preoperatively or prior to admission to hospital, as part of a policy to screen for possible unsuspected asymptomatic COVID infections.
The above study suggests that a weakly “positive PCR COVID test” (one that is positive only at a Ct greater than 35) in an asymptomatic person must be interpreted with caution.
In another study of screened patients, false positives were confirmed in 7.1% of ENT patients being routinely screened for COVID (with PCR testing) prior to urgently needed head and neck surgery. [18] That study involved only a small number of patients and must be interpreted with great caution. This study, however, like the Italian study, raises the possibility that in some settings, when people who are asymptomatic for COVID (or have only mild and non-specific symptoms) are screened for COVID with a PCR test, the false positivity rate might be higher than heretofore realized. Further study, though, is urgently needed to more definitively determine the false positivity rate in such settings.
“Live” virus vs “dead” virus: The PCR test does not detect only “live” virus. It also detects tiny amounts of harmless, inert, non-viable, non-contagious fragments of “dead” virus—viral debris. By “live” virus is meant viral genetic material that is capable of infecting cells (and replicating within those cells) and is, thereby, causing ongoing infection and is contagious. By “dead” virus is meant viral genetic material (typically small viral fragments) that is present but is inert and not capable of infecting (i.e., replicating within) cells and, thereby, is not contagious or causing ongoing infection. [2, 8, 9].
If the vast majority of the “119,238 daily new cases of COVID” were people who tested positive only at a Ct higher than 34, it is possible that the positivity of many of these people was due to presence of trace amounts of inert, non-viable, non-infectious viral material left over from past SARS-CoV-2 virus infection that had caused mild or no symptoms; and it is possible that many others were positive because of false positivity.
Ct results and viral culture: The best way to document the validity and significance of a positive COVID test would be to compare the PCR result with a viral culture of the same specimen. Viral culture is the “gold standard” for viral testing, in general—meaning that it is the most accurate way to determine presence of an active viral infection in a person and contagiousness of that person.
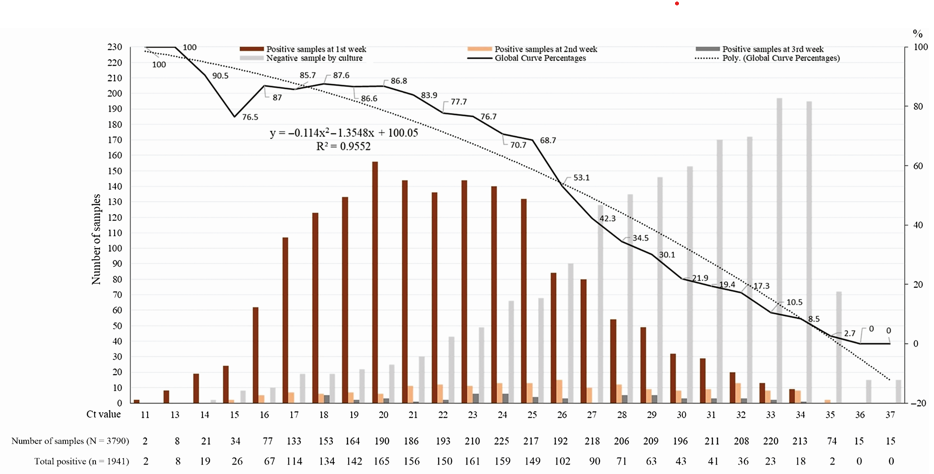
The Ct value correlates strongly (though imperfectly) with likelihood of culture positivity. [2, 8, 9, 24-26] In a study of ninety COVID PCR positive samples, twenty-six samples (28.9%) demonstrated viral growth, and all 26 of those samples had a Ct <25. [24] That study suggested that a Ct greater than 24 strongly correlated with reduced ability to culture the virus. [24] The odds of a positive culture decreased by 32% for each one unit increase in Ct. [24] In another study of 3,790 naso-pharyngeal samples, 70% of samples that were PCR positive at a Ct of 25 or less were culture positive, whereas <3% of samples that were PCR positive at a Ct of 35 or greater were culture positive, and none of the samples that were PCR positive at a Ct >35 were culture positive (see Graph 1 at end of References). [26]
Another study, however, points out the need for more caution and more study, regarding the relationship between Ct values and infectivity [25]. In that study, 8% of samples that were positive at a Ct> 35 had positive viral cultures. All those 8%, however, were symptomatic. In that same study, 6% of people who were more than 10 days post onset of symptoms were still culture positive.
At what Ct are people unlikely to be contagious? The extent to which a person with a positive PCR COVID test is contagious (i.e., capable of transmitting the virus to another person) correlates well (but imperfectly) with the Ct at which they are positive. [2, 8, 9, 24-26] The vast majority of contagious people are positive at a Ct less than 30, typically at a Ct in the lower 20s or upper teens. [8, 9] People who are positive only at a Ct of 37, 40, or 45 are very unlikely to be contagious, unless their testing happened to occur just before onset of symptoms (more on this later). [8, 9] If a person with known COVID has become asymptomatic and has a positive PCR test only at a Ct of 37 or higher, that person is not likely to have transmissible disease. [8, 9]
Although viral culture would be a far more accurate test for COVID and would inform infectivity more accurately than PCR, its use is limited because of its technical difficult, its labor-intensive nature, and the dangers (to lab personnel) associated with handling live virus. Accordingly, viral culture is impractical for large scale testing of COVID. Fortunately, Ct values, provide helpful guidance, regarding viral load and transmissibility.
Ct values change during the course of illness: Further complicating matters, the Ct value depends on timing and the location of the body from which the specimen is collected. [2, 3] During the first several days of symptoms (which typically start 5 to 7 days following exposure), the SARS-CoV-2 virus is present in largest amounts in the nasopharynx; then the viral load in the nasopharynx declines over the following week. Starting at about 7 days post-onset of symptoms, the viral load may be considerable in the lower respiratory tract, while being much lower in the nasopharynx—in which case testing sputum or a bronchoscopy specimen would be superior (either instead of, or in addition to, a nasopharyngeal specimen). So, timing and the location of sampling need to be taken into consideration.
Further comments about timing: The viral load begins to rise somewhere between 0-4 days before onset of symptoms; reaches a peak during the hours before onset of symptoms; declines dramatically during the first week of symptoms (thanks to our immune system); is low by the end of that week; then steadily declines thereafter. Infected individuals are typically contagious for about 7-10 days, starting hours before onset of symptoms. [8,9] Most are probably contagious for just a few days. [8,9]
A person’s Ct values will be inversely proportional to the viral load—so, the PCR COVID test will become positive at a high Ct at some point 0-4 days before onset of symptoms; then suddenly drop to a nadir (a much lower Ct value) during the hours before onset of symptoms (e.g., below 30, even well below 20) [8, 9, 25, 27]; then rise during the first week of symptoms (as the immune system decreases the viral load); then steadily increase thereafter, until the test becomes negative. A person’s PCR test may remain positive at a high Ct for many weeks (especially if a high Ct cutoff is being used for declaration of positivity), even 100 days, long after the person has ceased to be contagious, long after the virus has become “dead.” [2, 3, 8, 9]
Because of these timing issues, the PCR test may need to be repeated, if it is either obtained too early (just prior to the hours before onset of symptoms) or quite late in the disease course (after the viral load has diminished). If the PCR test is obtained too early, a timely repeat test will be more strongly positive. If the PCR test is obtained late in the course, repeat testing will be the same, less strongly positive, or negative. [8, 9]
Ct values and the severity of illness: Clinically, the Ct value at which a person’s PCR COVID test is positive correlates with the severity of illness and risk of dying. In a study of 678 hospitalized patients, 35% of those with a Ct <25 died: versus 17.6% of patients with a Ct of 25-30 and 6.2% with a Ct >30. [28]. Accordingly, when a person is informed that their COVID test is positive, it would be helpful and humane to let that person (and their physician) know whether they were positive at a Ct of 40, or at a Ct of 12.
The correlation between Ct value and illness severity is imperfect, however. In one study there was no difference in viral load across the spectrum of disease severity. [29] Some outpatients with minimal or no symptoms can have lower Ct values than severely ill patients in the ICU. In another study Ct values were lower (the viral load higher) in outpatients than in inpatients with worrisome COVID lung disease. [30]
On the surface, the above studies seem to contradict each other. However, many (even most?) patients with extremely severe COVID illness are severely ill, not because of a huge overwhelming viral load (at the time of severe illness), but because their immune system has greatly over-reacted to an average (or somewhat above-average) initial viral load—resulting in a harmful, life-threatening hyperinflammatory state, often with “cytokine storm.” Such patients may have a low viral load (high Ct value) by the time their life-threatening hyperinflammatory state (and most severe illness) has developed. Their immune system successfully diminished the viral load, but over-reacted in the process. At the other end of the spectrum are asymptomatic people who initially have a high viral load (low Ct value) but remain asymptomatic because their immune system quietly and efficiently eradicates the virus without over-reacting. These nuances need to be taken into consideration.
The importance of taking the following into account when planning care for a hospitalized patient: the patient’s viral load, the patient’s SARS-CoV-2 antibody status, the number of days since the onset of the patient’s COVID symptoms, and the usual time course of a hyperinflammatory reaction:
In a study of patients hospitalized for COVID-19 illness, 31.5% of patients had a positive COVID-19 PCR test at a Ct <22 on admission; 27% had a positive test at a Ct> 30 on admission; and 9.5% had a positive test at a Ct of 35 or higher on admission. This means that on admission at least 9.5% probably had little or no active infection and an additional 17.5% probably had only mildly active viral infection, at most. [31] At that same hospital a positive COVID PCR at a Ct of 22 correlated with a viral load of approximately 2,500,000 copies per mL. [32]
To supplement information provided by Ct values, it is helpful to document a patient’s SARS-CoV-2 antibody levels (IgM, IgG, or both). Antibodies to SARS-CoV-2 usually become detectable 1-3 weeks after onset of symptoms. According to one study, 32% of patients have developed at least low levels of IgG antibody within 4 days after onset of symptoms; by 7 days 48% have developed IgG antibodies; by 14 days 77% have IgG antibodies; and by 17-19 days 100% have IgG antibodies. [33] The antibody levels are lowest during the first week and highest during the third week. [33] If a patient has high IgG antibodies at the time of admission to the hospital or ICU, the clinician knows that the patient has been able to at least mount a good B cell response.
In the earlier-mentioned study of hospitalized patients [31], 38/114 patients (33%) had IgG SARS-CoV-2 antibodies at the time of admission. IgG antibody levels correlated inversely with viral loads. High viral loads almost never occurred in the presence of IgG antibody.
That same study [31] also showed that the viral load decreased as the days since onset of COVID symptoms increased. The vast majority of patients with a Ct less than 22 (low Ct, high viral load) were less than 7 days post onset of first symptoms. A minority of those with a Ct less than 22 were 7-10 days post onset of symptoms. Only a rare patient with a Ct less than 22 was 11-14 days post onset of symptoms. No patients who were more than 14 days post onset of symptoms had a Ct less than 22.
It is helpful to realize that the course of the viral load and the course of a hyperinflammatory/cytokine storm reaction are different and opposite. The viral load is highest during the hours before onset of COVID-19 symptoms and during the first few days after onset of symptoms. [8] By 7 days after onset of symptoms, the viral load is rapidly decreasing, due to the immune response. The viral load steadily declines thereafter. In contrast, the hyperinflammatory/cytokine storm reaction begins at some point during the first week and accelerates during the second and third weeks. As the viral load is declining, the hyperinflammatory reaction is accelerating. The viral load peaks early and usually subsides relatively quickly; hyperinflammatory reactions peak later and usually subside slowly.
If a patient is admitted to the ICU on day 12 (or later) with a new, unexplained surge of fever and/or other worsening symptoms, the timing alone would suggest a low viral load, presence of protective IgG SARS-CoV-2 antibodies, and presence of a hyperinflammatory/cytokine storm reaction. If such is confirmed, the patient would need immunosuppression, and it would be relatively safe to provide it. So, knowing the date of onset of a patient’s COVID-19 symptoms, the Ct value at which the patient’s admission PCR test was positive, the patient’s SARS-CoV-2 antibody status on admission, and the usual timing of viral versus hyperinflammatory phases of COVID illness, can help the clinician recognize whether a severely ill patient’s main problem is a hyperinflammatory reaction, or an ongoing high viral load, or both. Bear in mind that sometimes a hyperinflammatory reaction will subside spontaneously; but this cannot be relied upon. Usually, immunosuppressive medication is needed to control a threatening hyperinflammatory reaction.
So, knowing an admitted patient’s Ct value, IgG antibody level, the number of days post onset of initial COVID symptoms, and the usual time course of the viral and hyperinflammatory phases, helps the clinician to discern how high and how threatening the patient’s viral load is apt to be. This information, coupled with lab assessment of the extent to which hyperinflammation/cytokine storm is present (CRP, ferritin levels, etc.), helps the clinician to recognize whether the severely ill patient is suffering primarily from out-of-control active viral infection, excessive immune reactions to the virus, or both. This recognition, in turn, guides the clinician’s decision as to whether the patient primarily needs anti-viral therapies, or primarily needs immunosuppression, or needs both.
False positivity in the setting of surveillance testing: Again, the problem of false positivity is particularly important when it comes to mass screening of large populations of asymptomatic and minimally and non-specifically symptomatic people, as opposed to using the test in severely ill hospitalized patients who have a high pre-test probability of having COVID. In a severely ill hospitalized patient who has very typical COVID symptoms (i.e., has a high “pretest probability” of having COVID), a positive PCR COVID test is likely to represent a true positive, and very unlikely to represent a false positive. But, when asymptomatic (or only mildly and non-specifically symptomatic) people, who have a low pretest probability of having COVID, are screened for COVID, the likelihood of false positivity increases, especially if the prevalence of true COVID in the community is quite low, and particularly if the test is positive only at a high Ct.
If the false positivity rate is 4% and 1.5 million people are screened, all of whom are asymptomatic or minimally and non-specifically symptomatic, then 60,000 of those tested people would be expected to have a false positive test result. Those 60,000 people would not have COVID, they would have a false positive COVID test.
If the false positivity rate is 7%, and 1.5 million people are tested, 105,000 false positive results would be generated. If, two weeks later, 3 million people are tested, and the false positivity rate is 7%, then 210,000 false positive results would be generated, and this would give the false impression that the “number of new cases has doubled over a span of just two weeks!”
It is certainly plausible that the false positivity rate, when the test is used in a surveillance setting, is more than 4%. However, the fact is, we do not yet know the false positivity rate when the test is used in the surveillance setting—because this issue has not been sufficiently studied.
Revealing preliminary observations: Since at least July 2020, the average Ct value of people being diagnosed with “COVID” through COVID surveillance programs has reportedly been 35—at least according to one particularly knowledgeable virologist. [8, 9] In a study of three sets of PCR COVID test results (from Massachusetts, New York, and Nevada), 90% of people who tested positive “carried very little virus” (i.e., had high Ct values). [8, 9] For example, in July, in Massachusetts, where tests are declared positive at Cts of 37 or 40, 85-90% of people who tested positive would have been negative if the Ct cutoff had been 30. [8, 9] Further study of this issue is greatly needed.
The need for more study of Ct values: It will be important to determine the mean and median Ct values of the “positive tests” that are currently being generated by mass screening (of asymptomatic and mildly and non-specifically symptomatic people) and compare those data to the mean and median Ct values of hospitalized COVID patients—because these represent two different populations. If the mean and median Ct values of positive tests in screened asymptomatic people are greater than 35, this would suggest that those people are unlikely to be contagious—i.e., unlikely to transmit the virus to another person. Furthermore, it is possible that a significant percentage of these people (asymptomatic people with positivity at a Ct of 35 or higher, particularly at 37 or 40) have a false positive COVID test, not COVID. This possibility desperately and urgently needs further study.
Most current COVID PCR tests are still using a Ct of 40 as the cutoff value for declaration of a positive test. Some tests use a Ct of 37 as the cutoff, others use 45. Test results that are positive only at such high thresholds (Ct) are difficult to interpret with any certainty, especially in the surveillance setting. They may be simply detecting gene remnants of an old SARS-CoV-2 virus exposure—viral remnants that do not mean current ongoing active infection and are not capable of infecting other people—i.e., remnants that represent replication-incompetent, inert, non-viable, non-contagious viral debris (“dead nucleotides”). And it is also possible that many of these “positive” results (at high Cts) represent false positives, due to amplification of nonspecific off-target products (artifacts), and do not mean that the person is, or ever has been, infected with the SARS-CoV-2 virus. [22] A third, but much less likely possibility, statistically, is that the asymptomatic person may have been tested just before their infection was about to accelerate, in which case repeat testing over the next in 1-3 days would show a drop in the Ct value. [8,9]
So, if a person, especially an asymptomatic person who is participating in a screening process, is noted to have a “positive COVID test,” but it is positive only at a Ct of 35 or higher:
- They might have a tiny amount of true SARS-CoV-2 viral material in their specimen.
- That tiny amount of SARS-CoV-2 viral material would most likely represent inert, non-viable, replication-incompetent, “dead” remnants of virus that are not infectious/contagious. That is, if a viral culture were done, it would be negative, especially if the Ct is 37 or higher.
- It is possible that the person’s “COVID test positivity” is not even due to presence of any SARS-CoV-2 virus genetic material and, instead, represents a false positive. (As stated earlier, if the false positivity rate is as high as 4% and 1.5 million people are screened, then 60,000 of those tested people will be expected to have a false positive test. Those 60,000 people do not have COVID, they have a false positive COVID test.
- The least likely possibility is that the asymptomatic person happened to have been tested just before their infection was about to accelerate. This possibility can be evaluated by repeat testing over the next few days.
What would be the most appropriate Ct “cutoff” value? For the above reasons, a Ct of 30 (or 34 at the most) is generally recommended to be the most appropriate “cutoff” value for declaration of definite positivity. [8, 9] Using a Ct of 35, particularly 37 or 40 (and certainly 45) will result in far more positive results (including false positives), than if a Ct cutoff value of 30 or 32 is used—and, those with true positivity at a Ct of 37 or higher are very unlikely to be contagious. It would be reasonable to report “inconclusive positivity” at 37 or 40, as long as the significance of high Ct positivity is fully understood.
Ct results are not being routinely reported: To date, when “positive” test results are reported, the report has not routinely mentioned the Ct at which the test is positive—despite the above-mentioned value of knowing the Ct value. [2, 8, 9] Instead, the result is simply reported as “positive” or “negative,” with no additional details provided. Positive PCR test results are difficult to interpret, if there is no mention of Ct, and/or there is no information about the presence, absence, nature, timing, or severity of the person’s symptoms.
Limitations of the Ct value: Having just emphasized the importance of knowing the Ct value at which a person’s COVID test is positive, it is important to emphasize that interpretation of Ct values should be done with caution. [2, 3, 8, 9, 34] For example, the Ct value is affected by several variables, including the assay’s gene target(s), the extraction platform, PCR amplification chemistry, and the quality of specimen collection. Because of these variables, the Ct value at which a given person’s specimen is positive may vary, depending on the lab kit used and from lab to lab. As already mentioned, Ct values may also vary depending on the timing and location of sampling.
Furthermore, the Ct value provides only a relative indication of the viral load, not the actual viral load. All current COVID PCR tests have been designed as qualitative tests for COVID, not quantitative tests. They do not represent true quantitative tests for COVID. (True quantitative tests measure the actual viral load—i.e., copies per microliter.) Nevertheless, the Ct at which a COVID test is positive represents valuable information, gives a “fairly good’ indication of how large the viral load might be, and provides perspective. More data and more study are needed, however, to determine a more exact relationship between Ct values and actual viral load.
As already pointed out, additional data are also needed to more definitively determine the Ct and viral load levels at which COVID infectivity no longer occurs. And we should be careful to realize that viral culture negativity may not always mean non-infectivity. Some viruses are more difficult to culture than others.
What would be the most appropriate approach to surveillance testing and contact tracing? Perhaps the most appropriate and practical approach to surveillance testing and contact tracing would be to focus on detecting and acting upon those people who are most likely to be contagious (are positive at a Ct less than 30), and not try to detect all people who might have a tiny amount of inert, non-viable, non-culturable remnants of SARS-CoV-2 virus (people who are positive at a Ct of 37-40), because the latter are very unlikely to be contagious. [8]
How might serial Ct testing of hospitalized patients improve outcome? There is absolutely no question that COVID has life-threatening and life-taking potential, especially in the elderly and frail. A tragic number of people have died from COVID. We must protect the most vulnerable, and we must do all we can to save those who become severely ill with COVID.
There is a lot that we can do to try to save those with severe COVID illness. As explained in this article and two companion articles [35, 36], serial documentation of a patient’s viral load could improve the outcome of patients with severe COVID illness—by improving the selection, timing, and precision of needed treatments. Many people (a majority?) who die from COVID do so because their immune system has greatly over-reacted to the virus—resulting in a harmful, life-threatening hyperinflammatory state, often with “cytokine storm.” Others who die from COVID (a minority?) do so because of overwhelming viral infection, due to difficulty in slowing viral replication and lowering the viral load. In some patients both problems are present—hyperinflammation and overwhelming viral infection. In other patients only one of these problems (usually hyperinflammation) is present.
Life-threatening hyperinflammation requires prompt and aggressive life-saving immunosuppression (e.g., corticosteroid and anakinra); while overwhelming viral infection requires efforts to inhibit viral replication (anti-viral therapies). [36] Since it can be dangerous to give immunosuppressive treatment to patients who are carrying a large viral load, it is helpful to know, at least roughly, what a patient’s viral load is when administration of needed immunosuppression is contemplated. [36] The Ct value can provide a helpful estimate of viral load.
[Caveat: If a patient has been symptomatic for more than a week, the viral load may be higher in the lower respiratory tract than in the nasopharynx—in which case it would be better to perform the COVID PCR test on a specimen obtained from the lower respiratory tract (sputum or, ideally, bronchoalveolar lavage), rather than, or in addition to, the nasopharynx.]
For example, if, on admission to the hospital (or soon thereafter), a life-threatening hyperinflammatory state is noted and the patient’s COVID test is positive only at a high Ct (meaning the viral load has become low, thanks to a robust immune system), then needed aggressive immunosuppression can be provided promptly (while continuing to follow serial Ct values to make sure that the immunosuppression is not adversely interfering with viral eradication). On the other hand, if a newly hospitalized patient is noted to have both a hyperinflammatory reaction and a heavy load of active infection, then the patient needs both anti-viral therapy and immunosuppression, with administration of the latter being at least slightly delayed until suitable lowering of the viral load has occurred. If a newly hospitalized patient appears to be severely ill only because of overwhelming viral infection (high viral load) and has no evidence of hyperinflammatory/hyperimmune/cytokine storm reactions, then the patient may primarily need anti-viral therapies.
Careful serial monitoring (of viral load and evidence of a hyperinflammatory state) and timely (and accurately chosen) interventions are essential and may greatly improve the outcome of patients—i.e., decrease mortality and minimize irreversible organ damage in survivors. Such care may also decrease the duration of hospital and ICU stays and the need for mechanical ventilation, thereby reducing hospital overload and costs.
Please see companion article on Treatment of Patients with Severe COVID-19 Illness.
The importance of Ct values in the collection and interpretation of epidemiologic data (data regarding new and cumulative COVID cases, COVID hospitalizations, and COVID deaths):
As mentioned earlier, on 11/10/20 CNN reported that during the preceding week, in the USA [1]:
- 119,238 “new cases of COVID” were occurring per day (on average)
- “Soon, there will likely be 200,000 new cases occurring per day.”
- 59,000 “new COVID hospitalizations” were occurring per day.
- More than 1000 “new COVID deaths” were occurring per day.
Throughout the COVID epidemic the above kinds of epidemiologic data have been collected without any mention of the Ct value at which COVID tests were positive. The Ct value of positive tests have not been included in the data collected. It is also unclear what clinical criteria have been used to declare “new COVID cases,” “new COVID hospitalizations,” and “new COVID deaths.” The World Health Organization (WHO) and many government health ministries have encouraged diagnosis of SARS-CoV-2 infection (COVID) based on a single positive PCR result, even in asymptomatic persons without any history of exposure. For example, WHO has defined a “confirmed case” as a person with a positive test result, “irrespective of clinical signs and symptoms” and with no mention of Ct values. [37] Is that the policy that has been applied to the “new case” counts being currently reported in the USA?
In the USA, the Council of State and Territorial Epidemiologists (CSTE) has developed a more appropriate and nuanced “COVID-19 Interim Case Definition,” which was approved by the CDC in August 2020. [38] To what extent have the criteria recommended by the CSTE been strictly applied in the current collection, determination, and reportage of “new COVID cases” in the USA?
Neither the WHO, CDC, nor CSTE have recommended paying any attention to Ct values.
If most COVID tests are using a cutoff of 37 or 40 amplification cycles (a Ct of 37 or 40) to detect viral material; if most positive tests (especially in the surveillance setting) are positive only at a Ct of 35 or greater; and if the currently unknown incidence of false positive tests (especially in the surveillance setting) is higher than heretofore appreciated; then, the above reported COVID data may be greatly inaccurate—particularly “new case” data that have been generated primarily through mass screening of increasing numbers of asymptomatic and minimally (and non-specifically) symptomatic people.
Among many urgencies, there is urgent need to accurately determine the exact percentage of the “119,238 new daily cases” (and of future totals) that were positive only at a Ct of 37, 40, or 45, and had only been tested as part of a screening process. And it is essential to determine what percentage of “high Ct positive tests” represent false positives, particularly when this test is used in a surveillance setting.
It is possible that the CNN-reported data are roughly correct. However, it is also possible that:
- A high percentage (as high as 90%) of the 119,238 “new daily cases” represented people who were asymptomatic (or had, or had previously had, mild and non-specific symptoms) and, upon screening, had a “positive COVID PCR test,” but only at a Ct of 37 or 40, or 35 at the lowest. Ninety percent of 119,238 people would be 107,314 people.
- If the Italian study [17] is indicative of reality, 30% of those 107,314 people with a high Ct positive COVID test detected upon mass screening might be people with true positive test results (32,194 people), and the remaining 70% (75,119 people) might be people with false positive test results.
- The vast majority of the above-mentioned 30% with true positive results most likely contracted their COVID infection many weeks, even 1-3 months prior to their testing. Their test was most likely positive because the test was detecting trace amounts of inert, non-infectious, non-contagious viral debris (“dead virus”) left over from their previous exposure to COVID—meaning that these people were no longer actively infected or a threat to infect others.
If wise clinical criteria (such as that recommended by the CSTE) and wise laboratory criteria (including Ct values and appropriate interpretation of them) have not been strictly, uniformly, and accurately applied in the collection of data regarding “new COVID hospitalizations” and “new COVID deaths,” then these reported data (as well as the “new COVID cases” data) need to be interpreted with caution.
And the number of COVID deaths would be misleading if serial Ct values of severely ill patients have not been used to optimally guide selection and timing of treatment, and if patients who have developed hyperinflammation/cytokine storm have not received prompt, adequately aggressive immunosuppression.
Final comment about Ct values: Despite limitations and complexities, Ct values are important, helpful (clinically and epidemiologically), provide valuable perspective (regarding public education, public understanding, and public policy), and are a worthy subject of further attention and additional research.
COVID Antigen Testing:
Although this article has focused on PCR testing, brief mention of “rapid COVID antigen tests” is warranted. These tests detect antigens (proteins) on the surface of the SARS-CoV-2 virus. The US Department of Health and Human Services (HHS) recently purchased 150,000,000 of these tests and has been strongly promoting their use to screen school children and people in nursing homes to detect possible cases of asymptomatic or minimally symptomatic people who might be transmitters of COVID (i.e., for surveillance testing).
Unfortunately, the COVID antigen tests appear to be considerably less sensitive than the PCR test; and, thereby, generate a greater degree of false negative results. The antigen test also appears to generate more false positives than the PCR test.
For example, in Nevada, 23 of 39 “antigen positive” people (59%) proved to be negative upon the more accurate PCR test. [39] In Vermont, 65 positive antigen tests were noted, in mostly asymptomatic people. [39] When these same 65 people were tested with a PCR test, 60 of them (92%) were negative. The 4 who were positive by both antigen and PCR testing were symptomatic. In another study, 7 of 35 nursing home residents (20%) were noted to have a positive antigen test. But, when all 7 were tested by PCR, all 7 were negative. [39]
These preliminary experiences with antigen tests suggest that their use in surveillance testing (screening) is problematic—even more problematic than use of PCR tests in surveillance.
It is unclear what percentage of the “119,238 new cases of COVID” were positive only on an antigen test, versus being positive on a PCR test, or both. This information is essential in the interpretation of these data.
INSTRUCTIVE CLINICAL EXAMPLES:
With the above discussion in mind, let us imagine some instructive scenarios:
Persons A and B: Imagine that a healthy, asymptomatic 35-year-old male (Person A) dutifully gets a COVID PCR test, because two weeks earlier he was “exposed” to a friend (Person B) who, despite also being healthy and asymptomatic, recently had a “positive COVID test.” (Person B had gotten tested because he was planning to visit his grandmother and wanted to be certain about his COVID status.) Person A’s test comes back “positive.” Further investigation reveals that the tests employed for both Person A and Person B used a Ct of 40 as the cutoff for positivity. Both person’s tests were negative at Cts under 40, but positive at a Ct of 40.
Do Persons A and B “have COVID?” Do they represent “new cases” of either active or inactive COVID infection? Is their COVID positivity truly due to presence of COVID virus, at least a remnant piece of “dead” COVID virus? Possibly. Or, has one or both had a false positive test? Possibly.
Even if their tests have accurately detected presence of COVID-19 genetic material, what is the clinical significance of a test that is positive only at a Ct of 40 (suggestive of a copy count of less than 100 copies per microliter, as opposed to hundreds of millions of copies)? Does that mean the person is truly experiencing an active infection at the time of testing, is capable of “spreading” the virus, and needs to be quarantined for 14 days? Unlikely.
People who are positive at a Ct of 40 are unlikely to have active infection at that time, or to be contagious—unless the testing happened to have been done 1-3 days prior to onset of symptoms (i.e., just prior to the sudden increase in viral load that occurs during the hours before onset of symptoms), in which case they will become contagious at some point over the next 1-3 days. Even if their tests have accurately detected presence of COVID-19 genetic material (as opposed to representing a false positive result), is this positive result most likely a reflection of asymptomatic COVID exposure in the distant past, with a trace amount of harmless, inert, non-infectious, remnant COVID viral material still being present? Yes, this is much more likely, statistically, than the possibility that they are about to become infectious (with or without symptoms) at some point over the next 1-3 days.
Should Persons A and B be alarmed by their positive results? Should they be worried that they will soon become seriously ill? Should they be worried that they are a threat to others? The answer to these three questions is “no,” unless Person A’s testing happened to occur during the 1-3 days before the several hours just prior to onset of symptoms, which is unlikely and can be ruled out by watching for development of symptoms and repeating the test over the next 1-3 days. If the test has truly detected brand new infection, the Ct value will drop; if the test has detected old infection, repeat Ct values will be the same, increase, or the test will become negative.
Person C: Imagine Person C, a 72-year-old previously healthy female who, 2 months ago, experienced an ordinary “bad cold,” due to a confirmed common coronavirus infection. She fully recovered. Then, one week ago she developed typical “severe” flu-like symptoms (fever, chills, upper and lower respiratory symptoms, myalgias, arthralgias, malaise, and fatigue). Her symptoms rapidly worsened, necessitating hospitalization on day 2 of her illness. On admission a PCR COVID test was positive. Because she was “COVID test positive” and a resident of a retirement/assisted living institution in which several staff and residents had been noted to be “COVID test positive,” she was presumed to have COVID. She was not tested for influenza or other viruses, “because her COVID test was positive and testing for other viruses was, therefore, unnecessary.” Repeat PCR COVID tests on days 4 and 7 of her illness were also positive. It was not realized, however, that all three of her tests were positive only at a Ct of 40. (Her physicians did not think, or know, to ask for this information.) She went on to die (on day 7) of untreated cytokine storm, complicated by respiratory failure, stroke, and failure of other organs. Her death was attributed to COVID-19 and reported as such.
But did Person C truly die of COVID? Or did she die of severe unrecognized and poorly treated influenza B? Would a patient with such an acute and severe COVID infection have had positive COVID tests only at a Ct of 40 throughout the peak of her infection, if the illness were truly due to life-threatening COVID? What might her influenza test have revealed, if one had been obtained—a very high load of influenza B virus? Did her positive COVID test simply represent a false positive COVID test (possibly); or did her test simply detect a tiny amount of remnant COVID genetic material left over from unrecognized asymptomatic COVID exposure that occurred several weeks earlier (possibly); or, did her test detect a tiny amount of cross-reacting genetic material from the benign common coronavirus infection she had experienced 2 months earlier (possibly, but probably not—because cross-reactivity between COVID and common coronaviruses has, so far, never been documented with PCR COVID testing )?
Did she ever have any COVID infection? Possibly, but possibly not. Did she truly represent a “COVID case” (possibly, but, if so, probably just an asymptomatic case), a true “COVID hospitalization” (most likely not), and a “COVID death (most likely not)? Or did she die of severe influenza virus (most likely), with coincidental COVID test positivity that, itself, was either a false positive (possibly), or due to remnant fragments of distant asymptomatic COVID exposure (possibly), or due to distant cross-reacting common coronavirus infection (unlikely, but conceivable)?
Person D: Imagine Person D, a 55-year-old male with asthma and obesity who acutely developed upper and lower respiratory symptoms, required hospitalization, went into respiratory failure, and died. He had had contact with several “COVID positive” friends. His COVID test was positive at a Ct of 16, suggesting a high viral load. PCR tests for influenza A, influenza B, adenovirus, and rhinovirus were negative. He was not tested for common coronaviruses. His death was attributed to COVID-19.
COVID is almost surely the correct diagnosis for Person D. One could ask: how do we know with absolute certainty that he did not have a severe case of common coronavirus infection—with the positive COVID test being due to detection of cross-reacting genetic material of common coronavirus (rather than truly detecting COVID)? Such is unlikely unless certain test manufacturers have not carefully constructed their tests. To date, no cross-reactivity with common coronaviruses has been reported with any of the COVID PCR tests. So, he most likely represented a true COVID death.
Person E: Imagine Person E, a 90-year-old female resident of a nursing home who has had long-standing COPD, chronic partially compensated congestive heart failure, obesity, and depression. Three weeks earlier, during mass COVID testing at the institution (screening done “out of an abundance of caution”), she and several other residents had tested “positive for COVID.” She had no cold symptoms at the time and was otherwise in her usual state of health (ill-health). The same was true of the other residents who had tested positive. As a result of this testing, she and all other residents were quarantined in their rooms. This was the third time they had been strictly quarantined over the past 8 months. Throughout those 8 months they had been constantly quarantined to at least some degree.
Person E then suddenly became markedly short of breath. She steadily deteriorated over the next two days and died on day three of her acute illness. A repeat COVID test was performed on the day of her death and was again “positive.” By that time, she still had not noticed any flu-like or “cold” symptoms (sore throat, runny nose, fever, cough, myalgias). Her personal physician, who knew her well, was present at her bedside during the hour before her death. He attributed her death to obvious decompensated, long-standing congestive heart failure. He had checked on her two COVID test results and noted that both were positive, but both at a Ct of only 40. Since he had been instructed, by new CDC guidelines, to list COVID positivity (if it had occurred) on the death certificate, he dutifully listed “COVID positivity” on her death certificate.
A copy of her death certificate was sent to the state health department and the CDC, where she was reported to be a “COVID death.” Did she die of COVID (extremely unlikely)? Did she truly have COVID, at least at some point? Possibly, but alternatively, her positive tests could have been false positives. Even if she truly had had COVID, it was probably coincidental asymptomatic COVID infection (or exposure) that did not contribute to her death.
The above examples reveal how difficult it is to know with certainty what a “positive PCR COVID test” means, what a “new case of COVID” means, what a “COVID hospitalization” means, and what a “COVID death” means—particularly if the Ct is unknown and the “positivity” is not being interpreted in the context of other important, relevant, and complete clinical information. It is very likely that most, even all, of the currently available PCR COVID tests are fully capable of detecting only COVID genetic material and not genetic material from common coronaviruses. It is possible that a positive COVID test at a Ct of 40 is somewhat meaningful and helpful, in that it could mean that the person had asymptomatic or mild COVID infection or exposure at some time in the preceding several weeks or few months—or, less likely, that they are about to become symptomatic with COVID at some point over the next few days. But it is also possible that a positive test at a Ct of 40 represents a false positive—particularly if the test was done as part of a screening process or in an otherwise asymptomatic or minimally and non-specifically symptomatic person.
Nursing home scenario: One final instructive example: Imagine a large, well run retirement/nursing home with 300 residents (average age 83 years, range 70-105 years) and 100 staff members. Dutifully, out of an abundance of caution, plans are made to test all residents and staff for COVID on a weekly basis. None of the 400 people was symptomatic at the time of the first testing, but 16 of the 400 people (4%) had positive tests (8 staff and 8 residents), all at a Ct of 40 (unbeknownst to the institution, because Ct values were not reported, and no one knew to ask for them). An “outbreak” of COVID was declared. The “COVID positive” residents were strictly quarantined, the “COVID positive” staff were sent home for 14 days of quarantine, and the entire institution was placed on strict “lockdown.”
Five new staff were brought in to replace the 8 quarantined staff. The next week 12 more people were noted to be “COVID positive,” a different 12 (6 residents and 6 staff). All were positive at a Ct of 40. Those 6 staff were sent home for 14 days of quarantine, and 3 replacement staff were eventually hired. (It was becoming increasingly difficult to find caregivers during the pandemic, because many in the pool of potential caregivers had become “COVID positive;” some, despite being COVID test negative, fearfully opted out of the health care work force, to protect themselves and their families; and the wealthier nursing homes were able to attract available caregivers by offering higher pay than the less wealthy nursing homes could afford to offer.)
As the weeks went by, more and more residents and staff were noted to be “COVID positive,” despite the strict lockdown—always with positivity being at a Ct of 40 (still unbeknownst to the institution, which had never heard of Ct values and had no idea of the importance of Ct values). It became increasingly difficult to find and retain good caregivers. The institution became increasingly short staffed. The residents became increasingly isolated, lonely, frightened, depressed, anxious, inactive, demoralized, and less well cared for. Their own family members were not allowed to visit them. Many questioned whether such a life was worth continuing, especially those who were over 90 years old and already frail, tired, and in pain. Most no longer visited their physicians. They were either afraid to go to the hospital/clinic, or the hospital/clinic had partially shut down certain services, or the hospital/clinic was understaffed because many health care workers had “tested positive” and were on quarantine, or those over 90 stopped caring about their health.
Fairly soon, many of the nursing home residents, particularly those in their 80s and 90s, began to die, usually of their long-standing, sub-optimally monitored, gradually worsening co-morbidities, if not natural death from old age and/or loss of will to continue living. Some contracted seasonal influenza and died in the hospital, where they were presumed to have COVID, either because they had had a “positive COVID test” or because of their exposure to someone with a positive test. If a deceased resident had ever been “COVID positive” or had ever been “exposed to someone with COVID positivity,” these features were listed on their death certificate, and they were designated and reported (according to CDC guidelines) as “COVID deaths,” even if other medical problems were clearly the true cause of their death.
Bear in mind that all the “COVID positive” residents and staff members mentioned above were positive at a Ct of 40. None of them would have had a positive COVID test, if the Ct value for definite positivity had been set at 30 or 32. It is possible that many of the positive test results were false positives. Those with true positivity (at a Ct of 40) were extremely unlikely to be contagious. And it is possible that none of those who required hospitalization, or died, did so because of COVID infection. It is possible that many, even all, of those who died did so because of influenza, or other severe non-COVID respiratory infection, other non-COVID medical problems, or side effects of decisions made in response to “positive COVID tests” that were all positive at a Ct of 40.
If the COVID lab test used at the above fictitious institution had used a Ct cutoff of 30, none of the residents or staff members would have been diagnosed with COVID. Furthermore, if a Ct cutoff of 30 had been used at this institution, the associated low incidence of COVID in this high-risk institution would have been attributed (unknowingly) to strict lockdown measures, exemplary institutional leadership, and exemplary obedience and strict compliance on the part of the residents and staff. The institution would have been lavishly and publicly praised as an example of how strong leadership, individual personal responsibility and unselfishness, and disciplined compliance with strict lockdown measures can defeat COVID and save lives.
Moreover, at institutions that had used test kit with a Ct cutoff of 40, “outbreaks of COVID,” including many “COVID hospitalizations” and “COVID deaths,” would be attributed (incorrectly) to “poor leadership,” sub-optimal lockdown policies, lack of discipline and compliance on the part of residents and/or staff, individual selfishness, or combinations of these.
The institution using a Ct cutoff of 30 would be lavishly lauded, while the institution using a cutoff of 40 would have been unfairly shamed—all being unaware of Ct values.
SUMMARY/CONCLUSIONS:
On 11/10/20 CNN reported that over the preceding week [1]:
- 119,238 “new cases of COVID” were occurring per day (on average)
- “Soon, there will likely be 200,000 new cases occurring per day.”
- 59,000 “new COVID hospitalizations” were occurring per day.
- More than 1000 “new COVID deaths” were occurring per day.
The quality of the above data depends, fundamentally, on the quality, reliability, wise use, and wise interpretation of the COVID PCR tests upon which these data are based.
Optimal interpretation of a positive COVID PCR test requires an understanding of the Ct value at which the test was positive.
It is concerning that the Ct at which PCR tests were positive in the CNN-reported “new cases” was not taken into consideration and was not likely to have been made available.
If most COVID tests are using a cutoff of 37 or 40 amplification cycles (a Ct of 37 or 40) to detect viral material; if most positive tests (especially in the surveillance setting) are positive only at a Ct of 35 or greater; and if the currently unknown incidence of false positive tests (especially in the surveillance setting) is higher than heretofore appreciated; then, the above reported COVID data may be greatly inaccurate—particularly “new case” data that have been generated primarily through mass screening of increasing numbers of asymptomatic and minimally (and non-specifically) symptomatic people.
Among many urgencies, there is urgent need to accurately determine the exact percentage of the “119,238 new daily cases” (and of future totals) that were positive only at a Ct of 37 or 40, or 45, and had only been tested as part of a screening process. And it is essential to definitively determine what percentage of “high Ct positive tests” represent false positives, particularly when this test is used in a surveillance setting.
It is possible that the CNN-reported data are roughly correct. However, it is also possible that:
- A high percentage (even as high as 90%) of the 119,238 “new daily cases” represented people who were asymptomatic (or had, or had previously had, mild and non-specific symptoms) and, upon screening, had a “positive COVID PCR test,” but only at a Ct of 37 or 40, or 35 at the lowest. Ninety percent of 119,238 people would be 107,314 people.
- If the Italian study [17] is indicative of reality, 30% of those 107,314 people with a high Ct positive COVID test detected upon mass screening might be people with true positive test results (32,194 people), and the remaining 70% (75,119 people) might be people with false positive test results.
- The vast majority of the above-mentioned 30% with true positive results probably contracted their SARS-CoV-2 virus infection many weeks, even 1-3 months prior to their testing. Their test was most likely positive because the test was detecting trace amounts of inert, non-infectious, non-contagious viral debris (“dead virus”) left over from their previous exposure to the SARS-CoV-2 virus—meaning that these people were no longer actively infected or a threat to infect others.
If wise clinical and laboratory criteria (including Ct values) have not been strictly, uniformly, and accurately applied in the collection of data regarding “new COVID hospitalizations” and “new COVID deaths,” then these reported data (as well as the reported “new COVID cases”) need to be interpreted with caution.
In addition to improving the scientific quality of the above epidemiological data, attention to Ct values might markedly improve the clinical care of individual hospitalized patients—by improving the precision and timing of life-saving interventions. With the help of Ct values, many COVID deaths might be prevented.
Despite limitations and complexities, Ct values are important, helpful, and a worthy subject of further attention and research—especially regarding use of PCR COVID tests in mass screening of people who are asymptomatic or are (or have been) only mildly and non-specifically symptomatic, but also in the initial assessment and subsequent monitoring and treatment of hospitalized patients.
Bottom Line: Morbidity, mortality, hospital and ICU crowding, health care worker shortage (due to quarantining for a “positive test result,” or departure from the work force due to fear of contracting COVID), health care costs, public fears, public confusion, and individual angst might all be markedly decreased by paying attention to the Ct values at which tests are positive. Awareness of Ct values could greatly improve the quality of public education, public understanding, public dialogue, and public policy, regarding COVID.
More scientifically sound, higher quality epidemiologic data, including careful prospective collection and analysis of Ct data, are desperately needed. More scientifically sound, less polarized, better informed, more tolerant, more constructive dialogue and media coverage of COVID are desperately needed.
Robert Rennebohm, MD
rmrennebohm@gmail.com
12/14/20
REFERENCES:
- https://www.cnn.com/2020/11/10/health/us-coronavirus-tuesday/index.html
- Binnicker MJ. 2020. Challenges and controversies to testing for COVID-19. J Clin Microbiol 58: e01695-20. https://doi.org/10 .1128/JCM.01695-20
- Tom MR, Mina MJ. To Interpret the SARS-CoV-2 Test, Consider the Cycle Threshold Value. Clin Infect Dis. 2020 May 21: ciaa619. Published online 2020 May 21. doi: 10.1093/cid/ciaa619
- Hosseini A, Pandey R, Osman E, et al. Roadmap to the Bioanalytical Testing of COVID-19: From Sample Collection to Disease Surveillance [published online ahead of print, 2020 Oct 30]. ACS Sens. 2020; acssensors.0c01377. doi:10.1021/acssensors.0c01377
- Guo JJ, Yu YH, Ma XY, et al. A multiple-center clinical evaluation of a new real-time reverse transcriptase PCR diagnostic kit for SARS-CoV-2. Future Virol. 2020;10.2217/fvl-2020-0299. doi:10.2217/fvl-2020-0299
- Wang M, Chen D, Wu W, et al. Analytical performance evaluation of five RT-PCR kits for severe acute respiratory syndrome coronavirus 2 [published online ahead of print, 2020 Oct 27]. J Clin Lab Anal. 2020; e23643. doi:10.1002/jcla.23643
- Yüce M, Filiztekin E, Özkaya KG. COVID-19 diagnosis -A review of current methods [published online ahead of print, 2020 Oct 24]. Biosens Bioelectron. 2020; 172:112752. doi:10.1016/j.bios.2020.112752
- TWiV 640: Test often, fast turnaround, with Michael Mina. https://youtu.be/kDj4Zyq3yOA
- Your Coronavirus Test is Positive. Maybe it shouldn’t be. Interview with Michael Mina, MD. Published August 29, 2020; Updated September 17, 2020. https://www.nytimes.com/2020/08/29/health/coronavirus-testing.html
- Vandenberg O, et al. Considerations for diagnostic COVID-19 tests. Nat Rev Microbiol. 2020 Oct 14: 1–13. doi: 10.1038/s41579-020-00461-z
- Surkova E, et al. False-positive COVID-19 results: hidden problems and costs. Lancet Resp Med. published online, September 29, 2020. https://doi.org/10.1016/52213-2600(20)30453-7
- Mayers C, Baker K. Impact of false-positives and false-negatives in the UK’s COVID-19 RT-PCR testing programme. June 3, 2020. https://assets. publishing.service.gov.uk/government/uploads/system/uploads/ attachment_data/file/895843/S0519_Impact_of_false_positives_and_ negatives.pdf (accessed Aug 8, 2020).
- Skittrall JP, Wilson M, Smielewska AA, et al. Specificity and positive predictive value of SARS-CoV-2 nucleic acid amplification testing in a low-prevalence setting [published online ahead of print, 2020 Oct 14]. Clin Microbiol Infect. 2020; S1198-743X (20)30614-5. doi:10.1016/j.cmi.2020.10.003
- Hellou MM, et al Nucleic-acid-amplification tests from respiratory samples for the diagnosis of coronavirus infections: systematic review and meta-analysis, Clinical Microbiology and Infection, https://doi.org/10.1016/j.cmi.2020.11.002.
- Corman VM, Landt O, Kaiser M, et al. Detection of 2019 Novel Coronavirus (2019-NCoV) by Real-Time RT-PCR. Euro Surveill. 2020, 25 (3), 1−8.
- Corman V B, leicker T, Brunink S, et al. Diagnostic Detection of Wuhan Coronavirus 2019 by Real-Time RT-PCR; World Health Organization, 2020; pp 1−12.
- Francesca F, et al. Detection of SARS-COV N2 Gene: Very low amounts of viral RNA or false positive? J Clin Virol. 2020 Dec; 133: 104660. Published online 2020 Oct 14. https://doi.org/10.1016/j.jcv.2020.104660
- Katz AP, et al. False positive reverse transcriptase polymerase chain reaction screening for SARS-CoV-2 in the setting of urgent head and neck surgery and otolaryngologic emergencies during the pandemic: Clinical implications, Head Neck 42 (7) (2020) 1621–1628, https://doi.org/10.1002/hed.26317
- Wang Z, et al. External Quality Assessment for Molecular Detection of Severe Acute Respiratory Syndrome Coronavirus 2 in Clinical laboratories, The Journal of Molecular Diagnostics (2020), doi: https://doi.org/10.1016/j.jmoldx.2020.10.008.
- Cohen AN, et al. Diagnosing SARS-CoV-2 infection: the danger of over-reliance on positive test results; false positive test results impact clinical and policy decisions. medRxiv preprint doi: https://doi.org/10.1101/2020.04.26.20080911
- Cohen AN, Kessel B, Milgroom MG. Analysis of expected false positive rates in SARS-CoV-2 testing: technical background, limitations and objections. https://doi.org/10.5281/zenodo.4035317.
- Ruiz-Villalba A, et al. Amplification of nonspecific products in quantitative polymerase chain reactions (qPCR) Biomol Detect Quantif. 2017 Dec; 14: 7–18. Published online 2017 Nov 1. doi: 10.1016/j.bdq.2017.10.001
- Willman D. Contamination at CDC lab delayed rollout of coronavirus tests. April 18, 2020. https://www.washingtonpost.com/investigations/ contamination-at-cdc-lab-delayed-rollout-of-coronavirustests/2020/04/18/fd7d3824-7139-11ea-aa80-c2470c6b2034_story.html. (accessed Aug 16, 2020).
- Bullard J, et al. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin Infect Dis. 2020 May 22: ciaa638. Published online 2020 May 22. doi: 10.1093/cid/ciaa638
- Singanayagam A, Patel M, Charlett A, et al. (2020). Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin, 25(32), 2001483. https://doi.org/10.2807/1560-7917.ES.2020.25.32.2001483
- Jaafar R, Aherfi S, Wurtz N, et al. Correlation Between 3790 Quantitative Polymerase Chain Reaction–Positives Samples and Positive Cell Cultures, Including 1941 Severe Acute Respiratory Syndrome Coronavirus 2 Isolates, Clinical Infectious Diseases, ciaa1491, https://doi.org/10.1093/cid/ciaa1491
- https://www.sciencemag.org/news/2020/09/one-number-could-help-reveal-how-infectious-covid-19-patient-should-test-results
- Magleby R, Westblade LF, Trzebucki A, et al. Impact of Severe Acute Respiratory Syndrome Coronavirus 2 Viral Load on Risk of Intubation and Mortality Among Hospitalized Patients with Coronavirus Disease 2019, Clinical Infectious Diseases; ciaa851, https://doi.org/10.1093/cid/ciaa851
- He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020; 26:672-5. Doi:http://dx.doi.org/10.1038/s41591-020-0869-5.
- Yagci AK, Sarinoglu RC, Bilgin H, et al. Relationship of the cycle threshold of SARS-C0V-2 polymerase chain reaction and total severity score of computerized tomography in patients with COVID-19. International Journal of Infectious Diseases 101 (2020) 160-165.
- Bryan A, Fink SL, Gattuso MA, et al., SARS-CoV-2 viral load on admission is associated with 30-day mortality. Open Forum Infect Dis. 2020 Dec; 7(12): ofaa535. Published online 2020 Nov 3. doi: 10.1093/ofid/ofaa535
- Perchetti GA, Nalla AK, Huang ML, et al. Validation of SARS-CoV-2 detection across multiple specimen types. J Clin Virol. 2020; 128:104438. doi: 10.1016/j.jcv.2020.104438
- Long QX., Liu BZ., Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 26, 845–848 (2020). https://doi.org/10.1038/s41591-020-0897-1
- Rhoads D, Peaper DR, She RC, et al. College of American Pathologists (CAP) Microbiology Committee Perspective: Caution must be used in interpreting the Cycle Threshold (Ct) value. Clin Infect Dis. 2020 Aug 12; ciaa1199. doi: 10.1093/cid/ciaa1199. Online ahead of print.
- Rennebohm RM. Analysis of the COVID-19 epidemic: an additional narrative; an alternative response. Pediatrician (St. Petersburg). 2020;11(3):23-40. https://doi.org/10.17816/PED11323-40
- Rennebohm RM. Has undertreatment of severe COVID illness been widespread? A pediatric rheumatologist’s perspective. Russia Biomedical Research, 2020, Vol 5, No 3, p. 3-13.
- World Health Organization. Global surveillance for COVID-19 caused by human infection with COVID-19 virus. Interim guidance. 20 Mar 2020. https://www.who.int/emergencies/diseases/novelcoronavirus-2019/technical-guidance/laboratory-guidance.
- https://wwwn.cdc.gov/nndss/conditions/coronavirus-disease-2019-covid-19/case-definition/2020/08/05/ (CSTE Criteria)
- https://www.medscape.com/viewarticle/941054 (accessed on 11/17/20).
Graph 1: Percentage of positive viral culture of SARS-CoV-2 PCR positive Naso-pharyngeal samples from COVID-19 patients. No sample that was positive at a Ct >35 had a positive culture. Reference 26: Jaafar R, Aherfi S, Wurtz N, et al. Correlation Between 3790 Quantitative Polymerase Chain Reaction–Positives Samples and Positive Cell Cultures, Including 1941 Severe Acute Respiratory Syndrome Coronavirus 2 Isolates, Clinical Infectious Diseases, ciaa1491, https://doi.org/10.1093/cid/ciaa1491
0 Comments