Are SARS-CoV-2 Vaccines Safe, Effective, Necessary, and Wise?
Before embarking on mass administration of a vaccine, it is best to demonstrate that such vaccination is adequately safe, effective, necessary, and wise. Although the currently available COVID vaccines may prove to be adequately safe for most people, it is not yet known how safe, effective, necessary, and wise their use is.
Two other fundamental principles of clinical medicine are the importance of patient education and informed consent. Both are particularly important when people are offered treatments that have been incompletely studied and could, conceivably, have worrisome side effects.
With the above principles in mind, this article addresses concerns about the COVID vaccines—particularly the issue of safety.
Regarding safety:
Are the new SARS-CoV-2 vaccines, particularly the mRNA vaccines (the Pfizer and Moderna vaccines), adequately safe? Possibly. Unfortunately, we do not yet know. There are legitimate reasons to be concerned about the safety of these vaccines.
The two available mRNA vaccines are lipid-nanoparticle-formulated, nucleoside-modified mRNA vaccines that encode the receptor-binding domain of the spike glycoprotein of the SARS-CoV-2 virus.
Historically, during the development of mRNA technology, two major problems became apparent—unmodified mRNA is too immunogenic and too unstable to be clinically feasible [1]:
Unmodified mRNA proved to provoke a degree of immune reaction that rendered it unfeasible for clinical use. [1] For example, by stimulating Toll-receptors, unmodified mRNA adversely activates cells of the innate immune system and increases production of type 1 interferon and pro-inflammatory cytokines. It became necessary to modify the mRNA so that it would be less immunogenic. This was accomplished by incorporating pseudouridine into the mRNA. This nucleoside-modified mRNA proved to not only suppress unwanted mRNA-mediated immune activation, but also enhanced the stability and functionality of the mRNA. [1]
A potential problem with nucleoside-modified mRNA is that it apparently is less immunogenic because it dampens innate immune sensing. This suggests that it might be at least transiently immunosuppressive when injected into humans. This, in turn, raises the possibility that some people who receive the vaccine may develop at least a transient and possibly clinically significant decrease in immune competency shortly after vaccination. Indeed, in a phase I/II study of COVID-19 vaccine, healthy volunteers (under age 56) developed a transient, apparently clinically insignificant, dose-dependent lymphopenia during the first few days after initial vaccination (see Graph after References). [2] Whether interferon and cytokine levels also dropped is unclear, because this was apparently not studied (or at least not reported). These data raise the possibility that some elderly people, who may already be relatively less immunocompetent, may not tolerate this transient immunosuppression as well as younger, healthier people—i.e., the immunosuppression may be less transient and/or more clinically significant. It is conceivable that this could predispose some elderly people to develop a post-vaccination infection (by whatever infectious agents happen to be around).
So, one concern about the mRNA vaccines is that these vaccines may cause a transient immunosuppression that could render elderly people transiently more vulnerable to becoming infected (with whatever bacteria or virus they may be exposed to). It is possible, for example, that some of the deaths reported in some nursing homes after mass vaccination could be due to people dying from any one of a number of infectious agents because of transient vaccine-induced immunosuppression. After all, alert innate immune sensing, type 1 interferon, cytokines, and lymphocytes play major roles in our initial immune defense against infection.
Although eventual studies may prove that there is little or no need to be concerned about the above, this issue has not yet been adequately studied.
A second problem with the mRNA technology is that unmodified mRNA is normally quickly digested, before it has a chance to instruct the ribosomes to synthesize the desired protein (spike protein in the case of the COVID vaccine). So, to make the technology clinically feasible, the mRNA must be protected from digestion. This is done by encapsulating the mRNA in a lipid-nanoparticle. The lipid is polyethylene glycol (PEG). The lipid encapsulation protects the mRNA from digestion, makes it more stable, and enhances its entry into the cytoplasm of cells.
This raises the following unanswered question: Since it is protected, how long does this PEG-encapsulated mRNA remain in our cell’s cytoplasm before it eventually disintegrates? Does it persist for just hours, or for days? weeks? months? Do we know? This is important, because if the mRNA continues to instruct the ribosomes to make spike protein only for a matter of hours or days, that might be reasonable. But, if the PEG-encapsulated mRNA persistently instructs the ribosomes to keep making spike protein for weeks and weeks, this might create prolonged and harmful (not to mention unnecessary) immune reactions to this prolonged production of spike protein. In this way could the vaccine be predisposing some people to chronic immune reactions?
Although eventual studies may prove that there is little or no need to be concerned about the above, this issue has not yet been adequately studied.
This raises a third concern. We want a vaccine to provoke an appropriate immune reaction for an appropriate length of time. We do not want to provoke an excessive reaction or, a reaction that persists for an excessive length of time. It is unclear whether the mRNA vaccines provoke only an appropriate reaction (i.e., an appropriate level of antibody production, not too high or too low) and only for an appropriate length of time. Although eventual studies may prove that there is little or no need to be concerned about the above, this issue has not yet been adequately studied.
A fourth concern: How healthy is it to have PEG sitting in the cytoplasm of our cells? Do we know with certainty which cells of the body receive the vaccine’s PEG-encapsulated mRNA? Is it just the muscle cells near the site of injection? Probably not. Does the PEG-encased mRNA enter all cells that have a lipid cell membrane, including brain cells? Do we know the consequences of housing PEG in our various cell types? Although eventual studies may prove that there is little or no need to be concerned about the above, this issue has not yet been adequately studied.
A fifth concern: Apparently a significant percentage of people are allergic to PEG. Some such people may have allergic reactions, including potential anaphylaxis. How do we know who might have an initial or delayed allergic reaction to the PEG component of the vaccine?
A sixth concern: Are the mRNA vaccines setting people up for either transient or chronic autoimmune reactions? An example of this concern might be the Florida obstetrician who died of autoimmune thrombocytopenia shortly after receiving a mRNA vaccine. Shoenfeld et al have emphasized the capacity of the SARS-CoV-2 virus to trigger a variety of autoimmune reactions, including reactions occurring due to “molecular mimicry.” [3-5] “A massive heptapeptide sharing exists between the SARS-CoV-2 spike glycoprotein and human proteins.” [3] Please see Table 1. [3] There is a legitimate concern that the antibodies to spike protein that the mRNA vaccines train the immune system to produce might accidentally cross-react with peptides within normal human tissue (the phenomenon of molecular mimicry), either transiently, recurrently, or chronically. Although future studies might prove that the mRNA vaccines do not trigger significant molecular mimicry reactions, or other autoimmune reactions, this issue has not yet been adequately studied.
A seventh concern is the possibility that antibody dependent enhancement (ADE), or other similarly violent immune reactions, might occur in vaccinated people when, at a later date (many months, even years, later), they become infected with the wild SARS-CoV-2 virus. Historically, such reactions were seen when attempts were made to develop vaccines against the SARS-CoV-1, other coronaviruses, and RSV. [6-11]
For example, in an early preclinical trial of a SARS-CoV-1 vaccine, severe lung disease occurred in ferrets and monkeys who were given a whole virus vaccine and then challenged, later, with live SARS-CoV [8]. The lung sections revealed Th2-type immunopathology with eosinophils, suggestive of hypersensitivity. This pathology was similar to the Th2-type immunopathologic reaction seen in young children when they were given an inactivated RSV vaccine and were subsequently infected with naturally occurring RSV [9, 10]. Most of those children experienced severe disease when infected with wild RSV; two of the children died.
Feline infectious peritonitis coronavirus (FIPV) is an example of antibody-mediated enhancement (ADE) of virus uptake in macrophages, with resultant enhanced replication and dissemination of the virus, which results in enhanced disease severity [11]. Antigen-antibody complex formation and complement activation was documented in the FIPV model and can occur in some other coronavirus infections in animals.
Although future studies may prove that autoimmune reactions and ADE do not occur to any significant degree with the mRNA vaccines, these concerns have not yet been adequately studied.
An 8th concern is the possibility that spike protein-based COVID vaccines might promote development of more virulent and/or more transmissible strains of SARS-CoV-2, as the virus mutates to evade suboptimal immunity provided by the current vaccines. The current vaccines result in neutralizing antibodies to the SARS-CoV-2 spike protein. This likely provides at least partial and temporary protection (for the vaccinee), such that the vaccinee may experience little or no symptoms when they encounter the virus. However, the neutralizing antibodies do not eradicate the virus (in the vaccinee), and this allows opportunity for the virus to replicate (within the vaccinee) and develop mutations (while replicating in the vaccinee) that allow eventual viral evasion of the vaccinee’s neutralizing antibodies. The concern is that such vaccinees may then become primary asymptomatic spreaders of new more virulent and transmissible variants of the virus.
A related concern is that vaccinees, though initially protected from severe disease, may eventually (even soon) become vulnerable to viral variants with new, more tenacious spike proteins that are resistant to the vaccinee’s initial neutralizing antibodies. That is, vaccine resistance may develop. This would then require the vaccinee to receive a new vaccination against the new mutated spike protein. And a vicious cycle may occur that increasingly escalates the emergence of new more virulent and transmissible variants and need for new vaccination.
So, although current vaccines may provide at least partial and temporary protection for the individual vaccinee in the short term, these vaccines may create a worsening situation at the population level, both in the short and long terms. The individual vaccinee temporarily benefits, but the collective suffers, particularly unvaccinated young adults and children who become infected in the future with these ever-worsening strains. Eventually, the initial vaccinees suffer, as well.
An additional related concern is that the vaccine-induced neutralizing antibodies may, conceivably, compete with our natural immune mechanisms, thereby suppressing, confusing, or otherwise weakening our natural immunity. In general, natural immunity is superior to vaccine-induced immunity. These vaccine-induced neutralizing antibodies may, thereby, result in a less practiced and less protective natural immunity.
Although eventual studies may prove that there is little or no need to be concerned about the above conceivabilities, these 8th concerns have not yet been adequately studied.
In order to adequately address all of the above concerns, it will be necessary to study what happens to vaccinated (and unvaccinated) people over the course of at least 1-2 years post-vaccination, not just over the course of 2-3 months after vaccination. These vaccines have been rolled out for mass immunization after only very limited human studies had been conducted over the course of a few months. That is not a sufficient length of time to determine long-term safety.
We also need to remember that this is the first time in history that a mRNA vaccine has been used on humans. We are in the midst of conducting mass immunization of billions of people, while only beginning to study the safety of the administered vaccine. This is an uncomfortable plan, to say the least.
The above concerns raise the possibility that the currently available COVID vaccines (all of which are based on production of neutralizing antibody to spike protein) could make the COVID pandemic more dangerous for the vulnerable, rather than safer. Until the safety of these vaccines (both for individuals and the collective) has been adequately established, perhaps it would be wiser to slow down, largely leave development of immunity up to the human immune system, and focus, instead, on making peoples’ lives healthier (including getting rid of glyphosate and other environmental toxins) and working harder to successfully treat those who develop severe COVID illness?
Regarding Efficacy:
Are the COVID vaccines effective? Most likely, they will prove to be at least partially effective, at least temporarily. However, we do not yet know how truly effective these vaccines are or will be— both in the short term, with the current predominant strains; or in the longer term, with new strains. (See the 8th concern discussed above.)
According to the manufacturers, the mRNA vaccines are about “95% effective.” However, it is important for physicians and the public to realize that when the manufacturers state “95% efficacy,” they are, more accurately, referring to Relative Risk Reduction (RRR), not to Absolute Risk Reduction (ARR). According to good scientific practice, and according to FDA guidelines, reports of clinical trials data should include both RRR and ARR results. Unfortunately, when the New England Journal of Medicine (NEJM) published the clinical trials data for the two mRNA vaccines, and when the FDA granted EUA (Emergency use authorization) for both vaccines, the ARR was not mentioned. Both numbers are important. For some reason, neither the NEJM, the FDA, nor the press has reported ARR data.
The next question is: “What, exactly, is being reduced by 95% ? Does the “95% efficacy” mean a 95% relative risk reduction (RRR) regarding likelihood of: getting infected at all; getting infected with sufficiently high viral loads to be contagious (even if asymptomatic); becoming severely symptomatic; becoming hospitalized; needing ICU care; dying? Or was there a 95% RRR only for some of these outcomes?
The manufacturers’ data primarily provided RRR data on the number in each group (vaccinated versus placebo) who became COVID PCR positive, and secondarily provided data on numbers who developed “severe illness.” The reports did not include data regarding the extent to which infection and transmission were prevented. Since none of the patients in either arm died, protection from death could not be determined.
Unfortunately, variable COVID PCR test kits were used in these vaccine trials, tests were done at a variety of labs, and the Ct cutoff values were not mentioned. (See companion article on Ct values of COVID PCR tests.) Details were not provided, regarding the Ct values at which positive tests (in either group) had become positive. For example, in the placebo group it is important to know what percentage of positive tests were positive only at a Ct of 37, or higher and what percentage were positive at a Ct of 30 or lower? It is also important to know what the Ct values were in all patients with “severe” disease. These details and other clinical details are necessary for proper interpretation of the data.
Furthermore, a basic fundamental scientific principle is to establish that the vaccinated group and placebo group were truly comparable at entry into the study. For example, at entry, were the two groups equal regarding evidence of pre-existing immunity? At entry, were the two groups equal, regarding geographic location (including immediate neighborhood and the history of COVID in that neighborhood), housing situation, socio-economic conditions, degree of exposure to others with COVID, incidence of co-morbidities, incidence of COVID in the participants’ location (during the study period), and the incidence of new (possibly more virulent or more transmissible) strains in the participants’ immediate locations? Some of these factors were taken into consideration, but not all of them. Were all of those in the placebo group with “severe COVID” definitely ill with severe COVID, or were other factors responsible for the severity of their illness? These details are important.
Without all of the above details, it is difficult to know what “95% efficacy” truly means.
Historically, efforts to develop vaccines against SARS-CoV-1 and MERS were unsuccessful. It is much more difficult to create a successful vaccine for respiratory tract viruses than for systemic viral infections. On this basis alone, it would be surprising to have a COVID vaccine that is 95% protective against infection, disease severity, death, and transmissibility.
Regarding Necessity:
Because data on the number of COVID cases, COVID hospitalizations, and COVID deaths have been of low quality (see companion article on critical examination of COVID data), and because efforts to successfully treat those with severe COVID illness have been suboptimal (see companion article on treatment), we really do not know how desperately necessary this vaccine is. Add to this the limited evidence of efficacy.
The “urgent need” to provide these vaccines, despite lack of adequate study of safety, is a presumed “urgent need,” rather than an established fact, and it is a presumption that is based on low-quality data.
How Wise:
Since we currently have inadequate and incomplete information regarding the safety, efficacy, and necessity, of this vaccine, how wise is it to be currently vaccinating as many people as possible as quickly as possible? How ethical is it to try to vaccinate 7 billion people as fast as possible, before we know how safe, effective, necessary, and wise these vaccines are?
As mentioned earlier, until more data (and much higher quality data) become available, perhaps we would be wise to delegate the task of developing immunity to the SARS-CoV-2 virus primarily to the human immune system, and focus, instead, on “doing the important little things” and doing more to successfully treat those who develop severe COVID illness? With that in mind, please see the companion article, entitled: “An Interview with the Human Immune System.”
Informed Consent:
Finally, have the concerns discussed in this article been thoroughly explained to those who have already received the vaccine and to those who are anticipating vaccination? If not, should such explanation not be made available? Before they receive a 3rd “booster” dose (or a new vaccine tailored to match a latest variant) should people who obediently received 2 mRNA doses not be informed of the inadequately studied concerns mentioned in this article (and amplified in companion articles)?
REFERENCES:
- Karikó, K., Muramatsu, H., Welsh, F. A., Ludwig, J., Kato, H., Akira, S., & Weissman, D. (2008). Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Molecular therapy : the journal of the American Society of Gene Therapy, 16(11), 1833–1840. https://doi.org/10.1038/mt.2008.200
- Mulligan, M.J., Lyke, K.E., Kitchin, N. et al. Publisher Correction: Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 590, E26 (2021). https://doi.org/10.1038/s41586-020-03098-3
- Kanduc, D., Shoenfeld, Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine. Immunol Res 68, 310–313 (2020). https://doi.org/10.1007/s12026-020-09152-6
- Shoenfeld Y. Corona (COVID-19) time musings: Our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmunity Reviews 19 (2020) 102538. https://doi.org/10.1016/j.autrev.2020.102538
- Ehrenfeld M, et al., Autoimmunity Reviews, https://doi.org/10.1016/j.autrev.2020.102597
- Perlman S, Dandekar AA (2005) Immunopathogenesis of coronavirus infections: Implications for SARS. Nature Rev Immunol 5: 917–927.
- Tseng, C. T., Sbrana, E., Iwata-Yoshikawa, N., Newman, P. C., Garron, T., Atmar, R. L., Peters, C. J., & Couch, R. B. (2012). Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PloS one, 7(4), e35421. https://doi.org/10.1371/journal.pone.0035421
- Haagmans BL, Boudet F, Kuiken T, deLang A, Martina BE, et al. (2005) Protective immunity induced by the inactivated SARS coronavirus vaccine. Abstract S 12-1. Presented at the X International Nidovirus Symposium, Colorado Springs, CO.
- Castilow EM, Olson MR, Varga SM (2007) Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease. Immunol Res 39: 225–239. 33.
- Collins PL, Graham BS (2008) Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol 82: 2040–2055.
- Weiss RC, Scott FW (1981) Antibody-mediated enhancement of disease in feline infectious peritonitis: comparisons with dengue hemorrhagic fever. Comp Immunol Microbiol Infect Dis 4: 175–89.
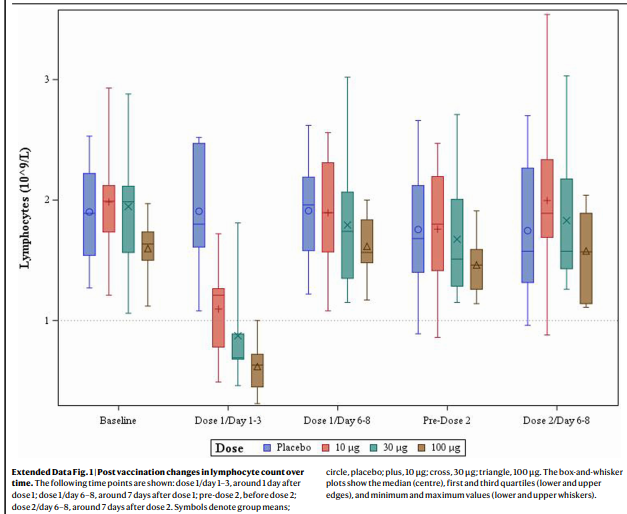
The above Graph is from is Reference #2, Mulligan, et al.
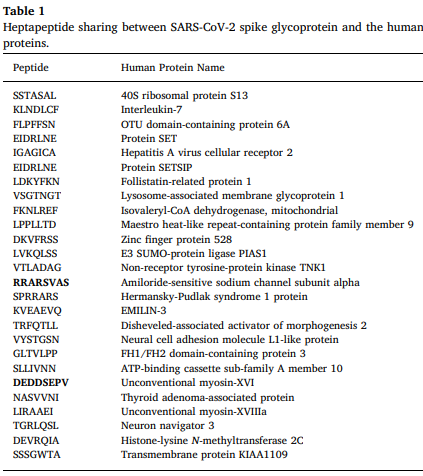
Table 1 is from Reference #3, Kanduc and Shoenfeld
0 Comments