An Open Letter to Parents and Pediatricians
Regarding COVID Vaccination
By Robert M. Rennebohm, MD
“I have tried to let Truth be my prejudice”
W. Eugene Smith, photojournalist (1918-1978)*

Two top priority goals during the COVID pandemic are to protect the vulnerable and provide the healthiest possible future for our innocent children. Does COVID vaccination give children the healthiest possible future; does it best protect the vulnerable? Or would a different approach to the pandemic give children a better future and better protect the vulnerable?

Is this boy crying because his grandparents have died of COVID? Is he worried about losing his parents to COVID? Is he frightened about dying from COVID himself? Is he sad that he has missed so much school, has seen so little of his friends, has not been able to play like before, and has had to wear a mask? Is he sad because his parents—one a physician, the other a nurse—have lost their jobs because they thought hospital policies were unscientific and harmful to patients? Is he worried that he may be forced to take a COVID vaccine that might cause him harm? Is he just very tired of worrying and hearing about COVID and all of the controversies and bad things going on in the world? Is he worried about the anxiety, sadness, and hardships people in his community have suffered because of COVID directives, particularly those who were already struggling emotionally and financially? Is he saddened by the cruel and intolerant ways in which people now frequently treat each other? Has he noticed that disagreements about COVID vaccination have torn families, friends, and communities apart? Does he wonder what happened to kindness, compassion, and healthy dialogue? Is he worried that these COVID issues will never end?

This image and the other two photographs are from the 1950s. If we were to fast-forward from then to 2022 and view this photo in the context of the COVID pandemic, what questions would cross your mind? What is he thinking? Is he thinking about his most recent patient who died of COVID and whether he and the hospital had done enough to save that patient? Is he thinking that his patient died from an adverse event caused by the COVID vaccine? Is he wondering what has caused this horrible COVID situation? Is he worrying about the overworked and exhausted staff at his hospital? Is he wondering whether this pandemic will ever end, whether we will learn enough from it, whether life will ever be the same, whether the practice of medicine is being forever changed, whether life will become increasingly worse?
*Eugene Smith:
The photographs at the beginning of this Open Letter are those of Eugene Smith.
W. Eugene Smith (1918-1978) was a brilliant, compassionate photojournalist whose work was most prominently shared in Life magazine during the 1950s and 60s. He cared deeply about issues of war, poverty, justice, suffering, and health care. Mostly, he cared about seeking truth, particularly social truths. And he longed for Social Beauty.
About his work, Smith said:
“With considerable soul searching, [and] to the utmost of my ability, I have let truth be the prejudice.
“If I can get them to think, get them to feel, get them to see, then I’ve done about all that I can as a teacher.”
We would all benefit if Eugene Smith were still with us today to chronicle the human suffering associated with the COVID pandemic—to help us to see, feel, think about, understand, and collaboratively resolve the COVID situation.
At the end of this Open Letter are two other Smith photos.
TABLE OF CONTENTS
Photographs by W. Eugene Smith
Table of Contents
Summary —-The Shorter Version of this Open Letter
Longer Version of this Open Letter:
Section 1: Two Conflicting COVID Narratives
Section 2: An Overview of the Human Immune System
Section 3: What Happens when a Pandemic is Managed without a Mass Vaccination Campaign?
Section 5: Other COVID Vaccine Concerns: Adverse Events
Section 6: Problems with PCR Testing and COVID Data Collection
Section 8: How Necessary and Wise has COVID Vaccination Been?
Section 9: Informed Consent—Obligations of Pediatricians
LINKS to Educational Video Interviews and Video Presentations
INTRODUCTION:
Two contradictory views on COVID vaccination have been expressed—a prevailing narrative (get vaccinated, immediately! Vaccination is our way out of the pandemic) and an alternative narrative (stop the COVID vaccination campaign, immediately! COVID vaccination is dangerous and making the pandemic worse). These conflicting narratives have created confusion and anxiety for parents and many pediatricians. This Open Letter is intended to help parents and pediatricians—who simply want to do the right thing—to better understand the science behind the conflicting narratives and decide on a best course of action, regarding COVID vaccination of children.
Informed Consent: Children have had no voice or vote, regarding their potential COVID vaccination. Children depend entirely on their parents to make a well-informed and wise decision. Ethically, experimental pharmaceutical products, particularly experimental vaccines that have been rushed into use before adequate testing for safety could be completed, must not be administered to anyone, particularly children, without adequate informed consent.
Pediatricians are legally and morally required to honor the principle of “Informed Consent” and make certain that parents are sufficiently informed before they (the parents) agree to have their children vaccinated. The information and concerns explained in this Open Letter represent the kind of information needed for a parent to make a well-informed decision before granting consent for COVID vaccination of their child.
Parents, I apologize for the length of this Letter. But the COVID vaccination issue is complex, deserves to be addressed more than superficially, and requires more than a simplistic understanding of immunology and vaccinology. Much is at stake. So, for the sake of your child and all children, please consider taking the time to read this Letter. If you don’t have time, consider reading just the SUMMARY—SHORTER VERSION OF THIS OPEN LETTER. (Next page)
As a pediatrician with nearly 50 years of experience, I have always believed in the importance of patient/parent education and the impressive ability of parents to adequately grasp complex medical information, particularly when their child’s health is at stake. Accordingly, I am confident that parents will be able to grasp the essence of information presented in this Letter.
At the end of this Letter, you will find over 1000 REFERENCES—almost all of which have either been published in peer-reviewed medical journals (the vast majority) or submitted as pre-prints for publication. Just before the References, you will find LINKS to several helpful Educational Video Interviews and Video Presentations.
If you would like additional information about COVID issues, please see the second section of my Notes from the Social Clinic website. It may be accessed by googling: https://notesfromthesocialclinic.org/ Twenty-one articles, covering most aspects of COVID, are posted in the second section of this Social Clinic website, starting with a Lead Article, entitled, “A Call for an Independent COVID Commission.” Here is the link to the Lead Article: https://notesfromthesocialclinic.org/a-call-for-an-independent-international-covid-commission/
SUMMARY— THE SHORTER VERSION OF THIS OPEN LETTER:
INTRODUCTION:
- Two contradictory views on COVID vaccination have been expressed—a prevailing narrative (get vaccinated, immediately! Vaccination is our way out of the pandemic) and an alternative narrative (stop the vaccination campaign, immediately! COVID vaccination is dangerous and has been making the pandemic worse).
- Unfortunately, there has been little or no healthy scientific dialogue between proponents of the two narratives, despite repeated pleas for such from leaders of the alternative narrative.
- These conflicting narratives have created confusion and anxiety for parents and pediatricians—who simply want to do the right thing. It has also created unhealthy division within the public, including rifts within families.
- This Open Letter seeks to: clarify the science behind COVID vaccination issues; facilitate healthy, inclusive dialogue; and bring people together to jointly determine what would be best for children and Humanity as a whole.
- The information and concerns explained in this Open Letter represent the kind of information needed for a parent to make a well-informed decision before granting consent for vaccination of their child.
Note: Over 1000 references for the statements made in this shorter version are embedded in the longer version. Please see the longer version for those references.
SECTION 1: TWO CONFLICTING COVID NARRATIVES:
- The Prevailing Narrative, promoted by the CDC and the US COVID Task Force, confidently states that the COVID vaccines are safe, effective, and necessary. According to this narrative: the pandemic has become a “pandemic of the unvaccinated;” the entire global population, with certain exceptions, must promptly become vaccinated, for the sake of protecting all of Humanity; COVID vaccination is a moral and social obligation of all citizens; to remain unvaccinated is selfish and socially irresponsible; and the alternative narrative (described in this Letter) represents harmful “misinformation.”
- The Alternative Narrative suggests that the pandemic has become prolonged and made more dangerous, not because of the unvaccinated, but because an unwise mass vaccination campaign that uses sub-optimal (non-sterilizing) vaccines is doing more harm than good. This alternative narrative represents a deep and sound scientific explanation that has been developed and articulated by extremely competent and caring scientists and physicians who have devoted their careers to the proper development and use of life-saving vaccines and have dedicated the past 22 months to thoroughly studying the COVID situation.
- According to the alternative narrative, on scientific grounds alone, the current COVID mass vaccination campaign must be urgently stopped—because the COVID vaccines are not safe, not adequately effective, and are harming people at both an individual and population level. According to this narrative, COVID vaccines should certainly not be administered to children.
SECTION 2: AN OVERVIEW OF THE HUMAN IMMUNE SYSTEM (HuIS):
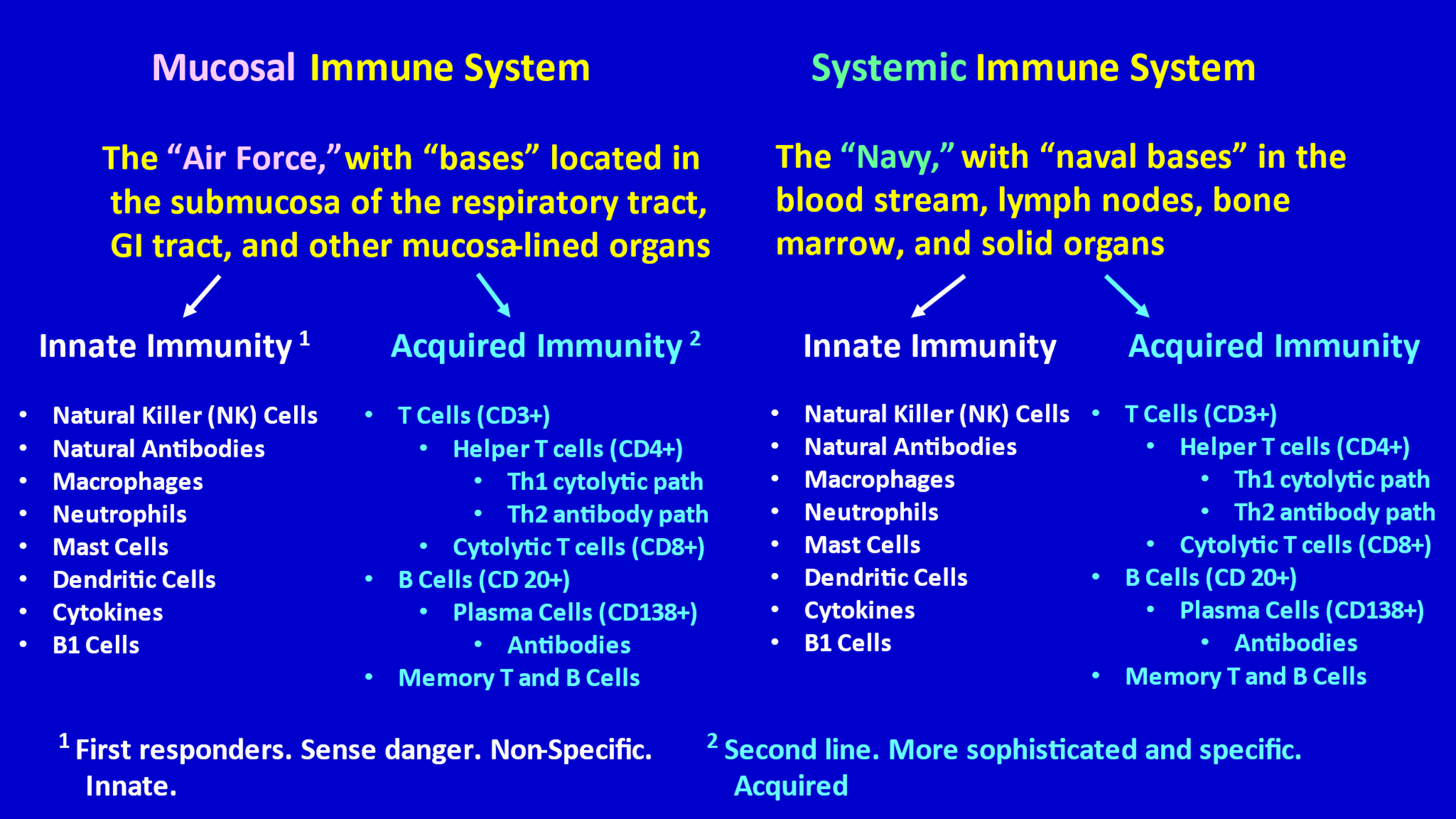
- The HuIS wisely approaches a virus in multiple ways. It does not simply and only produce specific antibody to a single major component of the virus, like the spike protein. It produces antibodies to multiple components up and down the virus, and it utilizes many other capacities in its vast armamentarium—innate immune capacities and acquired (adaptive) immune capacities.
- The immune system can be divided into two major compartments—the mucosal immune system and the systemic immune system. Dr. Bhakdi has helpfully referred to these two compartments as the “Air Force” (mucosal compartment) and the “Navy” (systemic compartment).
- The Air Force is “based” in the mucosa and submucosa (the space underneath the mucosal lining) of the respiratory tract, the GI tract, and the mucosa/submucosa of other mucous membrane-lined organs (e.g., bladder, uterus, etc.).
- The Navy is based (has “bases”) throughout the rest of the body—in lymph nodes, spleen, bone marrow, in the blood circulation, within solid organs, etc.
- Both the Air Force and the Navy have an Innate Immunity division and an Acquired (Adaptive) Immunity division.
- The mucosal immune system uniquely produces secretory IgA, which plays a key role in quickly combatting pathogens that enter the body through the respiratory tract or GI tract. The systemic immune system does not produce secretory IgA.
- In both the mucosal compartment and the systemic compartment, the first line of defense, the first responders, are the various troops of the Innate Immunity division, which includes natural killer cells (NK cells) and natural antibodies. If the innate immunity division determines that it is necessary to activate and mobilize the acquired immunity division (the second line of defense), it sends signals for that to happen.
- Sometimes only the Air Force (the mucosal immune system) is needed (particularly with viruses that enter through the nose and throat); sometimes only the Navy (systemic immune system) is needed; and sometimes both compartments (both the Naval bases and the Air force bases) must go into full action in order to protect a person from an invading virus.
- It is helpful to think of the immune system as an ingeniously orchestrated immune ecosystem, just like ecosystems in nature (forests, wetlands, etc.). Just as ecosystems in nature are complex, delicate, need to be respected, and must not be subjected to reckless tampering, the same is true with the immune ecosystem.
- In summary, when the SARS-CoV-2 virus invades a person, the HuIS potentially uses all of its multiple dimensions—both its mucosal immune system (the Air Force) and its systemic immune system (the Navy), both of which have an innate immunity division and an acquired immunity division—to quickly subdue the virus (initially by innate immunity troops of the Air Force) and create robust, durable, multi-dimensional acquired immunity to protect the person from future invasion by that virus.
- In comparison, the COVID vaccines provide uni-dimensional training of the systemic immune system and little, if any, training of the mucosal immune system.
- There is legitimate concern that the current COVID vaccines could be interfering with innate immunity and detrimentally disrupting the flow and optimal function of the natural human immune ecosystem.
- There is considerable scientific evidence that naturally acquired immunity is more effective, more robust, and more durable than the immunity provided by COVID vaccines.
SECTION 3: WHAT HAPPENS WHEN A RESPIRATORY VIRAL PANDEMIC, LIKE THE COVID PANDEMIC, IS NOT TREATED WITH A MASS VACCINATION CAMPAIGN?
- When a respiratory viral pandemic like the COVID pandemic is not treated with a vaccine (which was the case during the first year of the COVID pandemic, when no COVID vaccine was available), a considerable percentage of the population (primarily people under age 60, who are out and about) eventually becomes infected with the virus (the SARS-CoV-2 virus in this pandemic).
- The most vulnerable, including the elderly, must be carefully protected from exposure to the virus.
- Those who do become infected need to be proactively treated (much more promptly and aggressively than has been the case throughout the COVID pandemic).
- Those who become infected (and recover) develop robust naturally acquired sterilizing immunity that contributes to increasing development of herd immunity.
- The stronger the naturally acquired immunity of those who develop the disease (and recover) and the stronger the innate immunity of the “remaining” population, the faster herd immunity will develop and the stronger it will be.
- Once a high percentage of the population has developed naturally acquired immunity to the virus, herd immunity is well-established and strong, and the virus has a difficult time finding naive people (i.e., people who have not been infected and do not have naturally acquired immunity to the virus) to infect. As herd immunity increases and the number of naive people decreases, transmission decreases, and the pandemic subsides.
- So, the natural course of a respiratory virus pandemic is one of gradual resolution, usually over a period of months, and this resolution is largely due to increasing development of robust sterilizing herd immunity.
- The development of this herd immunity involves a dynamic and complex interplay between the virus and the immune system at a population level.
- It is important to understand that herd immunity via natural infection is far superior to herd immunity attempted via mass vaccination with a suboptimal (non-sterilizing) vaccine in the midst of an active pandemic. Herd immunity cannot be achieved through mass vaccination with a suboptimal (non-sterilizing) vaccine, and, In fact, such vaccination interferes with development of herd immunity. More on this later.
- The herd immunity that increasingly and cumulatively develops over the course of the pandemic protects everyone. In the meantime, appropriate public health measures and prompt and appropriately aggressive treatments minimize hospitalizations and deaths.
- Absolutely, the hospitalizations and deaths that occur are regrettable. However, according to the alternative narrative (and in my opinion as well), treatment of the pandemic with a mass vaccination campaign, using sub-optimal vaccines, results in considerably more cumulative hospitalizations and deaths than occurs in the absence of such a vaccination campaign, as explained in the next Section.
SECTION 4: WHAT HAPPENS WHEN A PANDEMIC LIKE THE COVID PANDEMIC IS PRIMARILY TREATED WITH A MASS VACCINATION CAMPAIGN, USING A SUB-OPTIMAL (NON- STERILIZING) VACCINE?
- The current COVID pandemic has been primarily managed with roll out of a rapid, mass vaccination campaign (across all age groups), using sub-optimal (non-sterilizing) uni-dimensional vaccines (directed at only the spike protein), in the midst of the active pandemic and in the midst of considerable lockdown measures.
- Unlike optimal (sterilizing) vaccines (which kill the virus and prevent infection and transmission) sub-optimal (non-sterilizing) vaccines do not adequately prevent infection or transmission. At best, they might lessen severity of illness.
- Proponents of the alternative narrative would favor use of an optimal COVID vaccine, if an optimal vaccine were available and proven to be safe and effective. But an optimal COVID vaccine has not been available.
- According to many experienced virologists/vaccinologists, a mass vaccination campaign using a sub-optimal (non-sterilizing) vaccine in the midst of a pandemic is a recipe for disaster. Why? Because, when a person who has been vaccinated with a sub-optimal vaccine is subsequently exposed to the virus, the vaccine does not prevent the virus from entering cells, replicating in those cells, and spreading to other people. And,
- When the virus replicates in the vaccinated person’s cells, new mutations develop, and under the pressure of the mass vaccination campaign and the added pressure of lockdown measures, the mutated variants that will be successful will be those that have an ability to “escape” the vaccine-induced anti-spike protein antibody and are more transmissible. The vaccine-induced antibodies quickly become less effective, the new variant more easily enters cells, more easily replicates, tends to be more transmissible than its predecessors, and can become an overwhelmingly predominant variant in the community. And,
- In addition to the concern that the COVID mass vaccination campaign will inevitably result in development of predominant variants with increased vaccine resistance and increased transmissibility, there is concern that the mass vaccination campaign might eventually generate a predominant variant that is intrinsically more virulent (deadly) than any of its predecessors—an intrinsically more virulent variant that could be harmful to everyone, including children, regardless of vaccination status.
- Even if increased intrinsic virulence does not occur, COVID illness may become more life-threatening because of vaccine-induced ADE.
- There is also concern that the COVID vaccines may be undermining and disrupting our normal immune system—particularly our innate immunity, particularly in children.
- Dr. Vanden Bossche, a leading proponent of the alternative narrative, disagrees that this is a “pandemic of the unvaccinated.” On the contrary, he views it as a pandemic that has become prolonged and more dangerous because of the mass vaccination campaign. Furthermore, he worries that it is the vaccinated people who are becoming the most likely “spreaders” of the virus—because the vaccine allows the vaccine-resistant variant to enter their cells and replicate, while the vaccine might indirectly make them less symptomatic, even asymptomatic, which results in their possibly being unwitting asymptomatic spreaders.
- Dr. Vanden Bossche thinks it is a huge mistake to continue the current COVID mass vaccination campaign. He strongly urges that we stop vaccinating before it is too late—before an even more transmissible and more virulent variant is generated (by mass vaccination), before ADE becomes more common, and before the immune systems of a higher and higher percentage of the population become compromised/hampered by the vaccines.
- He emphasizes that the greatest contributors to herd immunity are those who are unvaccinated and develop naturally acquired sterilizing immunity. If everyone becomes vaccinated, there will be no one left to develop unfettered naturally acquired sterilizing immunity.
- His admonition includes a plea to not start vaccinating people with a new vaccine that is directed against Omicron’s mutated spike protein. Such a vaccine would be only transiently beneficial, if at all, and would soon become obsolete when an inevitable new vaccine-resistant strain appears. Furthermore, continued vaccination with a new, updated vaccine will delay restoration of natural immunity that he fears has been disrupted and harmed by COVID vaccines. And continued vaccination will further delay development of herd immunity.
- The worst possible scenario would be the development of an extremely transmissible, more intrinsically virulent, vaccine-resistant strain that a fully vaccinated and ADE-prone population is unable to handle and that even unvaccinated children’s immune systems might have difficulty controlling.
- According to the alternative narrative, the total cumulative numbers of COVID hospitalizations, COVID ICU admissions, and COVID deaths during the COVID pandemic (from the beginning of the pandemic through January 2022) would have been lower if the pandemic had not been treated with the mass vaccination campaign and, instead, had been managed as described in Section 3.
- As with other issues raised in this Open Letter, it would be immensely helpful to establish an “Inclusive Independent International COVID Commission,” consisting of independent international panels of fairly selected, eminent scientists who would be asked to respectfully, thoroughly, objectively, transparently, and publicly address the conflicting points of view on COVID vaccination, in an effort to resolve disagreements and arrive at consensus.
SECTION 5: OTHER CONCERNS ABOUT THE COVID VACCINES—ADVERSE EVENTS:
- In addition to Dr. Vanden Bossche’s concerns that current mass vaccination is driving the development of more transmissible and potentially more lethal strains, may be harming natural innate immune function (particularly in children), and is interfering with development of sterilizing herd immunity, many scientists and physicians are deeply concerned that the COVID vaccines are unsafe in other important ways—causing unacceptable short- and long-term side effects for individuals.
- For example: myocarditis and pericarditis in adolescents and young adults; lethal clotting and devastating neurologic side effects in adults.
- The 1077 REFERENCES at the end of this Open Letter include 757 articles in the medical literature (the vast majority of them being peer-reviewed publications, the rest being pre-prints submitted for publication) that report serious side effects of COVID vaccinations (#271-1028). This represents an alarming and unprecedented number of reports of adverse effects of a new pharmaceutical product. Please consider scrolling through the REFERENCES and reading the titles of these 757 articles.
- The VAERS data also reveal an alarming number of severe adverse reactions and deaths associated with the COVID vaccines.
SECTION 6: PROBLEMS WITH THE COVID PCR TEST AND COVID DATA COLLECTED TO DATE:
- The prevailing narrative (its data, its conclusions, and its policies) has been fundamentally based on use of the COVID PCR test.
- A positive COVID PCR test cannot be adequately interpreted without knowing the Ct (cycle threshold) value at which the test was positive.
- If the test is positive at a low Ct value (e.g., 20), this means the specimen has a large amount of virus in it, the test is strongly positive, the diagnosis of COVID is more definite, and the person is highly contagious.
- A positive COVID PCR test at a Ct greater than 30 is likely to represent either a false positive (commonly) or detection of a tiny amount of dead virus. Many of such people have not, in fact, had COVID, and if they have had COVID, they are no longer infectious.
- When a COVID PCR test is positive at a Ct value greater than 27, the false positivity rate for the test is 75.5%. The higher the Ct value, the higher the false positivity rate.
- Unfortunately, the Ct values at which tests have been positive have not been shared with patients or their physicians.
- Even when a COVID PCR test is positive at a low Ct value, this does not assure that the patient definitely has COVID. The most accurate test for confirmation of COVID is genomic sequencing. Since the beginning of the pandemic, confirmed diagnoses of COVID should have been based on genomic sequencing, not on PCR testing.
- By basing data collection on the COVID PCR test and using it without disclosing Ct values and without genomic sequencing, the CDC and State Health Departments have generated scientifically unsound data.
- Making matters worse, data collection by the CDC and State Health Departments has been based on scientifically unsound criteria for designation of “COVID cases,” “COVID hospitalizations,” and “COVID deaths.”
- The scientifically unsound use of COVID PCR tests and the scientifically unsound criteria used for designation of “COVID cases,” “COVID hospitalizations,” and “COVID deaths” has resulted in US data being of low scientific quality, inadequately accurate, inadequately interpretable, and misleading.
- The prevailing narrative has not been based on proper conduct of science. This has been a huge and fundamental problem throughout the pandemic.
SECTION 7: EFFICACY OF THE COVID VACCINES:
- Proponents of the alternative narrative are concerned that the COVID vaccines are not nearly as effective as initially and subsequently claimed by their manufacturers.
- COVID vaccine failure would not be surprising, since: COVID vaccines are sub-optimal (non-sterilizing) and uni-dimensional; only partially train the systemic immune system; have little or no effect on the mucosal immune system; may be interfering with normal immune function; and drive the appearance and predominance of viral variants that “escape” the vaccinal antibodies and become increasingly transmissible and potentially more lethal.
- There are contradictory data regarding the extent to which the COVID vaccines are affecting infection rates, severity of illness, and incidence of death. This is not surprising, considering that the initial clinical trials and many of the subsequent studies of vaccine efficacy have been based on scientifically unsound data collection, as explained in Section 6.
- Several studies suggest that: the COVID vaccines actually increase risk of COVID infection and COVID death during the 5 weeks after the first dose; then there is temporary and modest protection (at best) for a matter of only weeks or a few months; then there appears to be a negative effect (increased susceptibility to COVID infection); and it is likely that Boosters will prove to provide only transient benefit, which is likely due to brief non-specific stimulation of natural immunity.
- Furthermore, there is legitimate concern that vaccine-induced ADE (antibody dependent enhancement) phenomena might be increasing disease severity and death in vaccinated people when they subsequently become infected; and there is some evidence that vaccinated people may be more likely to spread the virus than are the unvaccinated (because the vaccines may actually facilitate viral entry into cells).
- Several studies suggest that both the short-term effectiveness and the long-term effectiveness of the COVID vaccines are disappointing, especially with the newer variants. Naturally acquired immunity appears to be more robust and more durable than vaccine-induced immunity.
- For the reasons explained in Section 6, all vaccine efficacy data, from all institutions and all countries must be critically examined and interpreted with caution, including data from Johns Hopkins, WHO, the CDC and state health departments. Frankly, because of the problematic way in which COVID data have been collected to date, I do not think we know how truly protective the COVID vaccines have been.
- An Inclusive Independent International COVID Commission could helpfully assess the contradictory data and provide an appropriate consensus regarding the effectiveness of the COVID vaccines.
SECTION 8: HOW NECESSARY AND WISE HAS THE MASS COVID VACCINATION CAMPAIGN BEEN?
- The mass vaccination campaign, coupled with a failure to provide prompt and adequate outpatient and inpatient treatment, has resulted in a larger cumulative number of people with severe COVID illness and COVID death (during 2020 and 2021) than would have occurred if the COVID pandemic had never been treated with the current mass vaccination campaign and, instead, had been managed as described in Section 3.
- The mass vaccination campaign has appeared to prolong the pandemic and make it more dangerous. The vaccines themselves have caused an unacceptable number and degree of adverse events, at the individual level.
- There is legitimate concern and growing scientific evidence that the mass vaccination campaign has been unwise.
- Ideally, however, this issue should be addressed by an Inclusive Independent International COVID Commission.
SECTION 9: THE OBLIGATION OF PEDIATRICIANS TO PROVIDE SUFFICIENT INFORMATION FOR TRUE INFORMED CONSENT:
- Pediatricians, understandably, have had little time and energy to study the COVID situation in depth. (That is why this Open Letter, with its in-depth analysis and 1077 REFERENCES, is being offered.)
- Pediatricians are welcome to share this Open Letter to help adequately inform parents about COVID vaccination issues.
- The prevailing COVID narrative has been primarily based on scientifically unsound use of the COVID PCR test and scientifically unsound collection of data, regarding COVID cases, COVID hospitalizations, COVID deaths, and vaccine efficacy.
- Scientifically unsound data collection leads to scientifically unsound conclusions and scientifically unsound public policies.
- The alternative COVID narrative represents a deep and sound scientific explanation that has been developed and articulated by extremely competent and caring scientists and physicians who appreciate the complexity and elegance of the immune ecosystem, have devoted their careers to the proper development and use of life-saving vaccines, and have dedicated the past 22 months to thoroughly studying the COVID situation. They are not “anti-vaxxers.” On the contrary, they are pro-vaccination but insist that vaccines be adequately demonstrated to be safe, effective, and necessary.
- According to the alternative narrative, and in my opinion as well, the COVID vaccines are inadequately safe, inadequately effective, and have been doing more harm than good.
- The mass vaccination campaign, with its sub-optimal (non-sterilizing) vaccines, has prolonged the pandemic, generated more transmissible and potentially more lethal variants, has predisposed the vaccinated to life-threatening ADE, has transformed the vaccinated into major spreaders of the virus, has disrupted the normal healthy flow of immune system function (i.e., has harmfully disturbed the normal immune ecosystem, particularly the innate immunity of children), and has greatly interfered with development of sterilizing herd immunity. The result has been more deaths and hospitalizations than would have occurred if the COVID pandemic had been treated without this mass vaccination campaign.
- This has not been a “pandemic of the unvaccinated,” it has been a pandemic that has been prolonged and made worse by an ill-conceived mass vaccination campaign.
- In addition to being enormously problematic at a population level, the COVID vaccines have caused life-threatening side effects at the individual level.
- Furthermore, the COVID vaccines have been far less effective than claimed by their manufacturers and the CDC. At best they might provide transient benefit, and even that benefit is questionable.
- The risks associated with COVID vaccination (at both the population level and the individual level) appear to substantially outweigh the benefits—particularly in children and even in the elderly and frail.
- Moreover, throughout the pandemic, people who have become infected have commonly been undertreated—receiving neither proper early outpatient treatment, nor proper inpatient treatment. This has added to the hospitalizations and deaths that could have been prevented.
- The COVID vaccines have been promoted without proper informed consent. Neither the public, nor parents, have been provided adequate information by the proponents of the prevailing narrative—regarding vaccine side effects suffered by individuals and the adverse consequences of the mass vaccination campaign at the population level. Instead, they have been given simplistic, falsely reassuring, one-sided information and have been told that the information provided by the alternative narrative (the science-based, data-driven alternative narrative described in this Letter) represents “misinformation.”
- Parents and the public deserve to have representatives of the two opposing COVID narratives come together to engage in healthy, respectful, scientifically sound, publicly witnessed (televised) dialogue about the safety, efficacy, necessity, results, and wisdom of the current COVID mass vaccination campaign. For 22 months, leaders of the alternative narrative have been pleading for such, to no avail.
- Parents and the public can and should insist that an “Inclusive Independent International COVID Commission” be formed, consisting of independent international panels of fairly selected, eminent scientists who would be asked to thoroughly and objectively address COVID vaccination issues in an effort to resolve disagreements and arrive at consensus. Such is the tradition of science, medicine, democracy, and civil society. The public deserves and desperately needs such careful examination and healthy dialogue. Successful resolution of the COVID pandemic depends on it.
- Until the above-suggested Commission convenes and arrives at a thoughtful and fair consensus, the current mass vaccination campaign should—out of an abundance of caution—be at least temporarily suspended, at least for children.
- As parents and the public watch C-SPAN-like televised proceedings of the Commission, they (parents and the public) can decide in their own minds who among the Commissioners and discussants seems most knowledgeable, careful, rigorously scientific, compassionate, honest, ethical, and most wise.
- Only then will parents be able to make a truly informed decision for their children.
- As a pediatrician, a father, and a grandfather, I must do my part to protect the integrity of the immune ecosystem in children and to protect Humanity from the adverse effects of an ill-conceived mass vaccination campaign. Hence, this Open Letter.
- For the sake of our children, grandchildren, and all of humanity, we have an individual and collective social responsibility to call for an immediate and complete halt to the current COVID vaccination campaign, on a scientific basis alone, until an appropriate COVID Commission is convened to thoroughly and accurately evaluate the COVID situation. In the meantime, current scientific evidence strongly suggests that to participate in the continuation of the COVID vaccination campaign—-to promote it, to remain silent about it, or to personally receive further COVID vaccination—-is to contribute to the harm of children and humanity, as well as harm to oneself.
- Morally, ethically, and scientifically, we have a social responsibility to call for at least temporary cessation of the COVID vaccination campaign. Such a call is an unselfish, science-based act of courage and social responsibility, behind which all of humanity (whether currently unvaccinated or already vaccinated) can confidently unite, to the mutual support and the emotional, social, and health benefit of all.
THE LONGER VERSION OF THIS OPEN LETTER:
SECTION 1: THE TWO CONFLICTING COVID NARRATIVES:
The prevailing narrative: In the view of the CDC and the US COVID Task Force, the pandemic has become a “pandemic of the unvaccinated,” and “the way out of the pandemic” is for the entire population of the world to become fully vaccinated, including children. According to this narrative—which has been supported by most government leaders, most health care establishments, and almost all conventional media—the COVID vaccines are safe, effective, and necessary, and human beings have a social and moral obligation to become vaccinated, as soon as possible. According to this narrative, unvaccinated people are the problem—because they become infected; they allow the virus to replicate, mutate and spread to others; they become more ill than the vaccinated; and they disproportionately occupy hospital beds and resources.
An alternative narrative: In the view of a many other scientists and physicians—e.g., Dr. Geert Vanden Bossche, Dr. Luc Montagnier (Nobel Prize winner for discovering the HIV/AIDS virus), Dr. Sucharit Bhakdi, Dr. Mike Yeadon, Dr. Wolfgang Wodarg, Dr. Robert Malone (a major contributor to the development of mRNA technology), Dr. Byram Bridle, Dr. Michael Palmer, Dr. Peter McCullough, Dr. James Lyons-Weiler, and Dr. Paul Alexander—the pandemic has become prolonged and more dangerous, not because of the unvaccinated, but because a misguided mass vaccination campaign that uses sub-optimal (non-sterilizing) vaccines is doing more harm than good. These just-mentioned scientists and physicians are highly competent, extremely accomplished, deeply caring scientists and physicians with extensive knowledge and experience in virology, immunology, vaccinology, evolutionary biology, epidemiology, and evidence-based medicine. They are not “anti-vaxxers.” In fact, several of them have played major roles in developing life-saving vaccines, and all of them are pro-vaccine, as long as the vaccine has been adequately proven to be safe, effective, and necessary.
According to these thoughtful scientists, the current COVID mass vaccination campaign, strictly from a scientific standpoint, has been ill-conceived and must be urgently stopped—because the sub-optimal COVID vaccines are unsafe, inadequately effective, and are harming people at both an individual and population level—as will be explained later in this Open Letter. According to these scientists, currently available COVID vaccines should certainly not be administered to children. Dr. Vanden Bossche has thoroughly explained this alternative narrative via several video interviews—for example, see the three links below (as well as additional LINKS listed at the end of this Letter, just before the REFERENCES):
It is important to emphasize that, in the general population, proponents of the alternative narrative are a heterogeneous group who support it for varied reasons. The highly accomplished and experienced scientists mentioned above promote the alternative narrative primarily because they think it is based on sound science and sound medical ethics, and they feel strongly that the prevailing narrative is not based on sound science or sound medical ethics. Their support of the alternative narrative is based on the science and ethics involved, not on politics, ideology, or some other agenda. The political, economic, social, and religious views of these scientists and physicians are heterogeneous, not monolithic, and fall along a wide spectrum. In fact, some of these scientists have social views that are much farther “left” and much more progressive than the social views of US Democrats and Liberal Canadians who support the prevailing narrative.
Other proponents of the alternative narrative are not scientists or physicians. Some of these proponents appear to be motivated primarily by political and ideological concerns (e.g., a feared loss of individual liberty), rather than (or in addition to) scientific concerns, and some of these proponents espouse political, social, economic, and religious views that are very different from those of the above-mentioned scientists and physicians.
Although the prevailing narrative promotes the simplistic perception that supporters of the alternative narrative are all arch-conservative Republicans and right-wing extremists who are “anti-vaxxers,” “anti-science,” “anti-facts” and “deplorably spreading dangerous misinformation,” the reality is (the facts are) that there is considerable diversity and heterogeneity among proponents of the alternative narrative, and the alternative narrative, itself, represents a deep and sound scientific explanation that has been developed and articulated by extremely competent and caring scientists and physicians who have been pro-vaccination throughout their exemplary careers and have dedicated the past 22 months to thoroughly studying the COVID situation.
The need for healthy, scientific and public dialogue: Unfortunately, there has been little or no healthy scientific dialogue between the proponents of the prevailing narrative and proponents of the alternative narrative. Those who support the alternative narrative (most notably Dr. Vanden Bossche and Dr. Bhakdi) have repeatedly asked the supporters of the prevailing narrative to participate in healthy, inclusive, objective, transparent, publicly witnessed scientific dialogue about COVID issues—so that an accurate consensus can be reached and shared with the public. However, supporters of the prevailing narrative have avoided such dialogue and, instead, have summarily dismissed supporters of the alternative narrative as being harmful “spreaders of misinformation.”
This lack of healthy, inclusive scientific dialogue is antithetical to the proper practice of science and medicine. This lack of adequate scientific dialogue has, in turn, led to a lack of healthy and inclusive public dialogue about COVID. Great polarization has resulted within the public, even within families, particularly over the issue of vaccination. This contentious polarization has left parents and pediatricians in the difficult position of not knowing whom to believe, whom to trust, and what to do—particularly about vaccination of children. Parents and pediatricians desperately want to do the right thing, both for their children and for society, but the right course of action has been unclear.
A call for healthy and inclusive dialogue: As a pediatrician, a pediatric rheumatologist, and a grandfather, I offer this Open Letter in hopes that it will help clarify the science behind COVID vaccination issues, facilitate healthy and inclusive dialogue, and bring people together to jointly determine what would be best for children and Humanity as a whole. Since the US COVID Task Force and the conventional media have abundantly and exclusively presented their prevailing narrative and have largely dismissed, demonized, and even censored the alternative narrative—such that the public has become disproportionately familiar with the prevailing narrative and (in many cases) strongly prejudiced against the alternative narrative—I will try to correct that imbalance by giving appropriate voice to the alternative narrative.
SECTION 2: AN OVERVIEW OF THE HUMAN IMMUNE SYSTEM (HuIS):
Before we delve deeply into issues of COVID vaccination, let us review how the human immune system normally and naturally protects us from infection. I apologize for the length of the explanation that follows, but it is essential for parents, physicians, and the public to have a general appreciation (even if only vague) of the ingenious complexity, beauty, flexibility, wisdom, delicacy, and collaborative nature of normal human immune system function, as well as the complexities involved with vaccination. It is not necessary for the reader to fully grasp the information in this section. What is important is to appreciate the multi-dimensional elegance of the immune system and how that compares to and is potentially disturbed by COVID vaccine-induced immunity.
I want to emphasize that the explanation that follows simply represents my best current understanding of the immune system and how it works. There may well be aspects of this understanding that will need correction—at least eventually, as we continue to learn more about the complexities of our immune system. As with other information presented in this Letter, it would be best to engage leading experts in immunology, virology, vaccinology, epidemiology, and evolutionary biology in objective, careful, healthy, inclusive, publicly witnessed, peer-to-peer dialogue about the kind of information I am about to present. Such peer-to-peer dialogue would either confirm the accuracy of that information or provide important improvements/corrections of it.
In the meantime, here are my understandings:
The human immune system (HuIS) normally has tremendous capacity and uses great wisdom to deal with viral infections, including those as new and threatening as SARS-CoV-2. The HuIS wisely approaches a virus in multiple ways. It does not simply and only produce specific antibody to a single major component of the virus, like the spike protein. It produces antibodies to multiple components up and down the virus, and it utilizes many other capacities in its vast armamentarium—innate immune capacities and acquired (adaptive) immune capacities; mucosal capacities and systemic capacities.
The Figure below provides an overview of the immune system and might help you to keep things straight, as you read the following written explanation of how the immune system orchestrates its many components and capacities to protect us from infection:
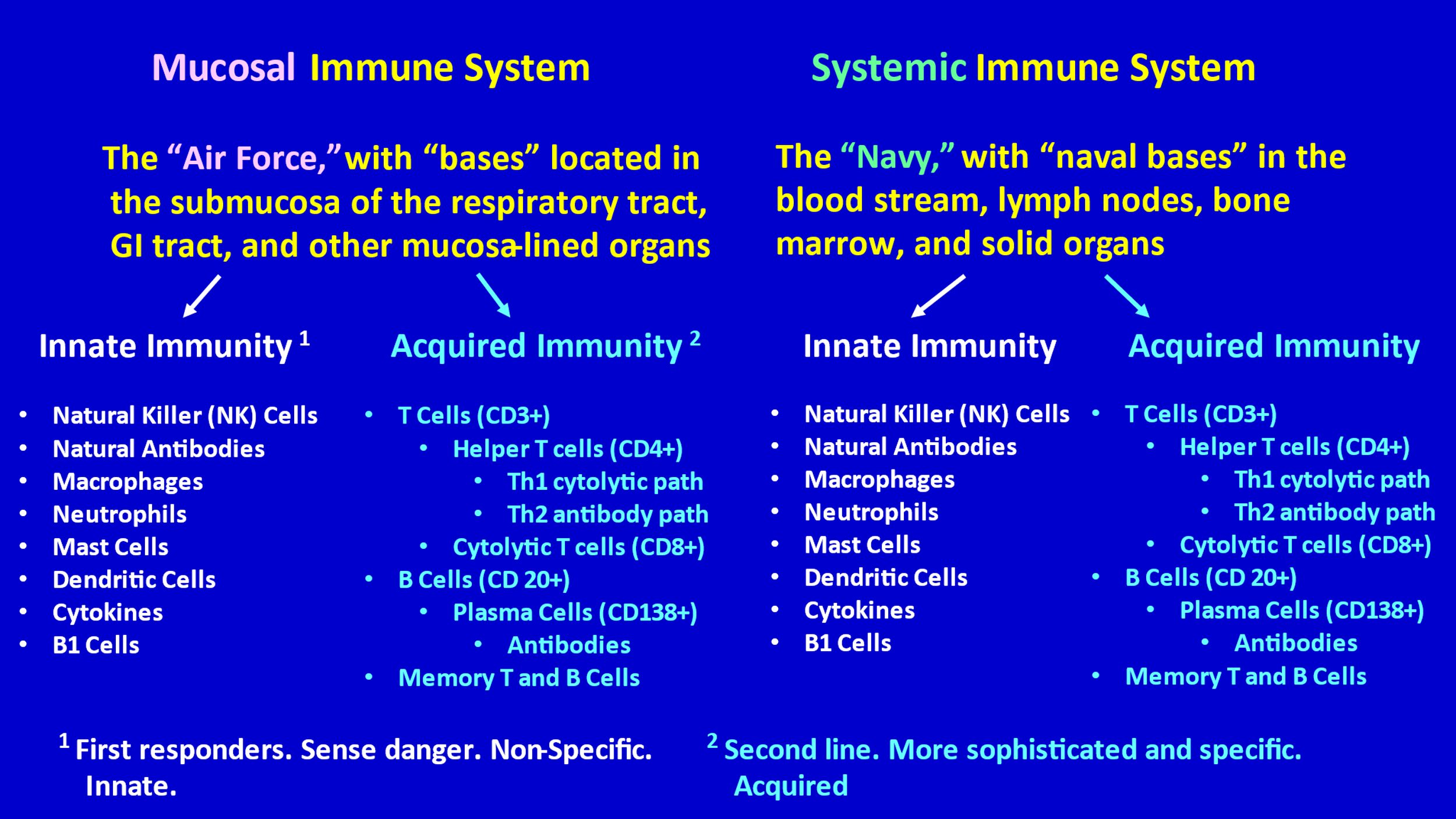
First, it is helpful to know that the immune system can be divided into two major compartments—the mucosal immune system [1-10] and the systemic immune system. Dr. Sucharit Bhakdi has helpfully referred to these two compartments as the “Air Force” (mucosal compartment) and the “Navy” (the systemic compartment). (See LINK L.) The Air Force (or the “border patrol” might be a better name) is “based” in the mucosa (and submucosa) of the respiratory tract (upper airways and bronchi), the mucosa of the GI tract, and the mucosa of other mucous membrane-lined organs (e.g., bladder, uterus, etc.). The Navy is based (has “bases”) throughout the rest of the body—in lymph nodes, spleen, bone marrow, in the blood circulation, within solid organs, etc.
The mucosal immune system is actually the larger compartment of the human immune system—much larger than the systemic component. [2] This makes sense, because infectious agents, particularly during childhood, primarily enter the human body through the respiratory tract and gastrointestinal tract, both of which are lined by mucosa. A prompt and effective immune response by the mucosal immune system can prevent infectious agents from invading the systemic compartment of the human body. The mucosal immune system is particularly active and important in children, who are frequently coming into contact with respiratory and gastrointestinal infectious agents that are new to them.
A major difference between the mucosal immune system and the systemic immune system is that the mucosal immune system can produce secretory IgA antibodies, while the systemic immune system does not. Secretory IgA is a particularly important early defense measure. More on this later.
Both the Air Force and the Navy have an Innate Immunity division and an Acquired (Adaptive) Immunity division.
Innate Immunity: The Innate Immunity division consists of a variety of “troops” that are born with certain important capacities. Some of these troops have the ability to recognize dangerous intruders (like viruses). They announce to the rest of the immune system that they have spotted a threat (danger), and they act as first responders. Other troops are natural killer cells (NK cells) that quickly recognize infected cells and kill those cells—which, in turn, kills the virus inside those cells, thereby preventing spread of viral infection. Other troops (B1 cells) produce innate “natural” non-specific antibodies (mostly large IgM antibodies) that attach to invaders (like viruses) in a loose and non-specific way and enable recognition of virus-infected cells by NK cells, thereby abrogating the infection of cells. [11-16]
These “natural antibodies” are non-specific in that they are capable of recognizing and attaching to a diversified spectrum of potentially dangerous viruses that have invaded, but they are not capable of recognizing exactly what specific virus has invaded (for example SARS-CoV-2 versus some other coronavirus). Fortunately, to do their job these natural antibodies do not need to know exactly what specific virus is present. In the mucosal compartment, for example, they capture and eliminate viruses that target mucosal cells; it’s only when the virus breaks through this first line of immune defense that a more specific immune response (by the acquired immunity division) will be mobilized against the specific invading virus.
Other troops of the innate immunity division include macrophages, dendritic cells, and neutrophils. Among other things, these cells gobble up and digest invaders.
The various troops of the innate immunity division communicate among themselves and with troops of the acquired immunity division via Cytokines. Cytokines are messenger molecules that activate and energize components of both the innate immunity division and the acquired immunity division. Excessive release of cytokines, however, can result in a “cytokine storm,” which is a potentially harmful hyperimmune/hyperinflammatory reaction.
Children, compared to adults, have particularly strong innate immunity, though it is initially immature. [17] Because children are young and healthy, and because they frequently encounter a wide variety of infectious agents, especially during the first 5 years of life, their innate immunity division develops lots of valuable experience during early life (especially in the mucosal compartment). Not only are they born with a robust innate immunity division, but their innate immunity becomes even stronger due to frequent practice and self-training during childhood. This “training” is thought to be a process of epigenetic changes. We should try to avoid unnecessarily interfering with this valuable practice and training.
Acquired (Adaptive) Immunity: The acquired (adaptive) immunity division is the second line of defense. The troops in this division have more sophisticated and specialized abilities to attack specific viruses. They acquire their specialized abilities only through experience with the specific virus involved One platoon of B cells (antibody producing cells) in this division acquires an ability to produce (and then only produces) specific antibodies that bind only to a specific virus (like SARS-CoV-2). Another platoon of B cells learns to produce specific antibodies to a different specific virus, like an influenza virus. And so on. These capacities are not innate. These B cells are not born with this ability to produce antibody to a specific virus. They acquire this learned ability only after first experiencing the specific viral infection involved.
The acquired immunity division also has troops that are capable of specifically attacking and killing cells that have become infected with a specific virus. These are virus-specific cytolytic T cells (CD8+). They are like the innate immune division’s natural killer cells, except that they are targeted at pathogen-specific peptides that are presented on the surface of infected cells.
The orchestrating cells of the acquired immunity division are helper T cells (CD4+). Some helper T cells (Th2 cells) help B cells, by activating these B cells so that they (the B cells) start producing their specific antibodies. Other helper T cells (Th1 cells) help by activating specific cytolytic T cells. Helper T cells know whether and when they need to activate B cells and/or cytolytic T cells.
A key ability of the acquired immunity troops is that they are capable of specific, long-lasting memory. After the troops of the acquired immunity division react to a first encounter with a specific virus, they are able to remember that experience and are able to quickly mount a similar specific B cell antibody response or a specific cytolytic T cell response to that same specific virus if/when that virus invades again, even when invasion occurs many years later. This is called B cell memory and T cell memory; it is specific for a specific virus, and it is long-lasting. The innate immunity division also has capacity for memory, but it is not as sophisticated and specific as the memory capacities of the acquired (adaptive) immunity division, and it is thought to be epigenetic in nature. It is important to note that on exceptional occasions T cells may acquire cytotoxic properties without acquiring immunologic memory. It is thought that this is, for example, the case in people who rapidly and spontaneously recover from COVID.
So, to review, when a respiratory virus enters the respiratory tract, the first line of defense, the first responders, are the troops of the Innate Immunity division of the mucosal immune system. If the innate immunity division of the mucosal immune system determines that it is necessary to activate and mobilize its acquired (adaptive) immunity division, it sends signals for that to happen. Sometimes the innate immunity division of the mucosal immune system can eradicate a viral infection without needing to activate its acquired immunity division. It depends on how robust the innate immunity division is and how threatening the infection is. If the mucosal immune system senses a need to activate the systemic immune system, it sends signals for that to happen.
It should be realized that sometimes only the Air Force (the mucosal immune system) is needed; sometimes only the Navy (systemic immune system) is needed; and sometimes both compartments (both the Naval bases and the Air force bases) must go into action in order to protect a person from an invading pathogen. For example, when a virus (e.g. SARS-CoV-2, other coronaviruses, influenza virus, or RSV) or a bacterium enters the respiratory tract, the Air Force immediately goes into action—either the Air Force’s innate immunity division (e.g., in the case of viral infection at the mucosa) or the Air force’s acquired immunity division (e.g., in the case of extracellular bacterial infection at the mucosa) successfully fight the pathogen at the portal of entry. Innate immune effector cells synergize to capture pathogens that hide in mucosal/epithelial cells (e.g. viruses), whereas mucosal IgA deal with pathogens that colonize mucosal surfaces outside of mucosal/epithelial cells. The Air Force may suffice to control the invading pathogen or may require the Navy (the systemic immune system) to be mobilized. If a mucosal pathogen threatens to break through the Air Force’s defenses or in case a pathogen bypasses the mucosal immune defense system and immediately invades the systemic circulation/compartment and threatens other organs in the body, then the Air force certainly and quickly signals the Navy to become active. The Navy then mobilizes its innate and acquired immunity divisions. Mobilization/activation of the Air Force and/or Navy may enable the immune system to control the infection and leads to immunologic training and/or memory of innate and acquired immune effector cells, respectively.
Production of Secretory IgA by the submucosal immune system: As mentioned earlier, an important and unique capacity of the mucosal immune system is its ability to promptly produce large quantities of secretory IgA, which then coats the mucosa and neutralizes invading pathogens. [1, 8, 9] In fact, secretory IgA is produced in quantities that are far greater than those of all other immunoglobulins that the human immune system produces. [3] The secretory IgA is produced by B cells within the submucosa/mucosal tissues. [4] An important difference between secretory IgA and other immunoglobulins (like IgG and IgM antibodies) is that secretory IgA is able to neutralize pathogens without creating a potentially harmful inflammatory reaction. [1] IgG, for example, tends to trigger considerable inflammation, which is often necessary and helpful, but can be harmful.
IgA can also be produced by the systemic immune system, but this systemic (or circulatory) IgA is different from secretory IgA. [1] Systemic IgA is produced in the bone marrow and does not get effectively transported onto mucosal surfaces. [5] So, systemic IgA does not participate in topical mucosal immune protection.
Limitations of systemic vaccination: Finally, it should be realized that systemic vaccination (intra-muscular administration of vaccine, as is the case with the COVID vaccines) trains the systemic immune system but provides little, if any, training of the mucosal immune system and, therefore, does not beneficially affect mucosal immune function. [6] In fact, there is concern that the vaccine’s training for a systemic immune response might not be optimal for inducing an immune response to a respiratory virus (which is invading the respiratory compartment of the body, not the systemic compartment, and, therefore, needs the Air Force more so than the Navy). More on this later.
The Human Immune Ecosystem: The above-described immune system—its two major compartments (the mucosal immune system and the systemic immune system) and the innate and acquired immunity divisions within each compartment—is an ingeniously orchestrated, marvelously performing system that has developed and perfected its extraordinary, coordinated capacities over thousands of years. It is an extremely complex, efficient, collaborative system, with many checks and balances, finely tuned and orchestrated. I like to think of the immune system as an elegant immune ecosystem, just like the precious ecosystems in Nature. Just as ecosystems in Nature (forests, wetlands, prairies, lakes, etc.) are complex, delicate, need to be respected, and must not be subjected to reckless tampering, the same is true with the human immune ecosystem.
Environmentalists and ecologists know, too well, how quickly and disastrously Nature’s ecosystems can be damaged and disrupted by arrogant and igNORant tampering by those who erroneously think their interventions will only benefit and not cause harm. There are too many examples of human tampering that has resulted in regrettable environmental/ecological disaster. For example, too often, giant mining, timber, and agricultural corporations have intolerantly and dismissively refused to listen to the concerns of environmentalists, have disrespectfully scoffed at the concerns of ecologists, and have continued to autocratically ravage the environment. We (including giant pharmaceutical companies) need to treat the human immune ecosystem with the same respect and care that we need to treat environmental ecosystems.
In summary, when the SARS-CoV-2 virus invades a person, the HuIS potentially uses all of its dimensions—both its mucosal immune system (the Air Force) and its systemic immune system (the Navy), both of which have an innate immunity division and an acquired immunity division—to quickly subdue the virus (initially by innate immunity troops) and to create robust, trained innate immunity and durable acquired immunity to protect the person from future invasion by that virus.
Naturally Acquired Immunity versus Vaccine-Created Immunity: Because of the elegant and ingenious complexity of the human immune system—its multi-faceted, multidisciplinary, multi-dimensional, collaborative approach; its diversity, division of labor, respect for and use of all aptitudes; its flexibility, adjustability, efficiency, wise checks and balances, feedback mechanisms and back up mechanisms; its ability to learn from experience; its practiced training and astonishing memory; and the fact that its capacities have been perfected over thousands of years—most immunologists, virologists, and vaccinologists agree that naturally acquired immunity is superior to vaccine-induced immunity, at least when compared to COVID vaccine-induced immunity. There is a great amount of evidence that naturally acquired immunity to SARS-CoV-2 is superior to the immunity provided by the current COVID vaccines. [18–163] This, in part, is because the human immune system (HuIS) approaches the virus in a multi-dimensional way, starting with a rapid and effective response by the mucosal immune system in the respiratory tract.
In contrast, the COVID vaccines are uni-dimensional (they are focused only on the spike protein of the virus, not on other components of the virus) and they reach and train only the systemic compartment of the immune system, not the mucosal compartment.
It is a shame that the COVID vaccines do not train or give practice to the mucosal immune system, because the SARS-CoV-2 virus enters the body through the respiratory tract (and possibly through the GI tract) and often never penetrates into the systemic compartment—thanks to the mucosal immune system’s ability to usually contain the virus within the respiratory tract. If the COVID vaccines were capable of fully training and mobilizing the mucosal immune system, they would be much more effective than they are. The main offering of the COVID vaccines is partial training of the systemic immune system, so that it (the systemic immune system) can respond if the mucosal immune system fails to contain the virus within the respiratory tract and the virus invades the systemic compartment. Even when that penetration does occur, the multi-dimensional approach of the natural systemic immune system is much more effective than the uni-dimensional (spike protein-based) response that the COVID vaccine teaches.
Furthermore, as will be discussed later (Section 4), the COVID vaccines are sub-optimal (non-sterilizing) vaccines—meaning that they do not fully prevent virus from infecting our cells, and they do not prevent transmission of the virus from one person to another. Optimal (sterilizing) vaccines prevent infection of cells and prevent transmission.
Moreover, there is legitimate concern that vaccinal COVID antibodies might detrimentally interfere with natural antibodies and other natural multi-dimensional responses of the naturally behaving immune system. This concern includes the possibility that vaccinal antibodies might interfere with the training and practice that a young child’s innate immunity division needs and normally gets in the absence of interfering vaccinal antibodies. In other words, the COVID vaccines might be harmfully disturbing and disrupting the normal immune ecosystem, particularly in children. That is why it is so important to appreciate the complexity and delicate balances within the natural immune ecosystem and avoid reckless tampering with it. For more information about disturbance of innate immunity and natural antibodies by COVID vaccination, see Dr. Vanden Bossche’s video interviews, either by clicking on the Vanden Bossche LINKS at the end of this Letter (LINKS A-I and O), or by clicking on the following website: https://www.voiceforscienceandsolidarity.org/
Also, “An Interview with the Human Immune System” may be found at this link: https://notesfromthesocialclinic.org/interview-with-the-human-immune-system/
SECTION 3: WHAT HAPPENS WHEN A RESPIRATORY VIRAL PANDEMIC LIKE THE COVID PANDEMIC IS NOT TREATED WITH A MASS VACCINATION CAMPAIGN?
When a respiratory virus pandemic is not treated with a vaccine (which was the case during the first year of the COVID pandemic, almost all of 2020, when no vaccine was available), an increasing percentage of the population becomes infected with the virus (the SARS-CoV-2 virus in this pandemic). The spread of infection will be slower or faster, less extensive or more extensive, depending on many factors—including the intrinsic infectiousness (transmissibility) of the virus; the extent to which strict mitigation policies (strict isolation, physical distancing, etc.) are deployed; the extent to which over-crowding, poor nutrition, and other health threatening adverse socioeconomic and environmental factors are at play; the extent to which anti-viral therapies are optimally deployed; the overall health (including immune system health) of the population; and the age of the population (which is important because the elderly have less competent immune systems, more co-morbidities, and, therefore, are at greatest risk of serious illness and death).
If the elderly and most vulnerable are adequately protected, the largest group to become infected will be the relatively young adults (those under age 60), because they are out and about and are most likely to be exposed to the virus. It is less worrisome when the relatively young and healthy (as opposed to the elderly and frail) are the ones who become infected, because they are the best able to withstand the infection with least harm (thanks to their strong immune systems and better overall health, as well as thoughtfully prescribed treatments and other common sense protective measures), and most will do well, particularly if treated appropriately (see later paragraphs).
When people in this younger age group (those under 60) become infected, their natural innate immune system and their natural acquired immune system come to their aid, eliminate the virus (within those who are infected), render them non-contagious, and minimize their transmission of the virus to others. When they recover, they have robust and durable naturally acquired sterilizing immunity that has completely eliminated the virus (in the infected individual). Infection of this younger group, and the naturally acquired sterilizing immunity resulting from their infection, increasingly contribute to development of robust herd immunity at the population level, which then serves to increasingly protect the elderly and vulnerable. Early in the pandemic, the percentage of the population that has developed naturally acquired sterilizing immunity is low, but as the weeks and months pass that percentage increases and eventually reaches sufficiently high levels to protect the population as a whole. The stronger the naturally acquired immunity of those who develop the disease (and recover) and the stronger the innate immunity of the “remaining” population, the faster herd immunity will develop and the stronger it will be.
Once a high percentage of the population has developed naturally acquired sterilizing immunity to the virus, herd immunity is well-established and strong, and the virus has a difficult time finding naive people (i.e., people who have not been infected and do not have naturally acquired immunity to the virus) to infect. As herd immunity increases and the number of naive people decreases, transmission decreases, and the pandemic subsides.
So, the natural course of a respiratory virus pandemic is one of gradual resolution, usually over a period of months, and this resolution is largely due to increasing development of robust herd immunity. But it is more complex than this, and that complexity is important to at least briefly discuss. During the course of the pandemic there is a dynamic and changing interplay between the virus and the immune system at a population level. For one thing, during the course of the pandemic, many viral mutations occur, and more infectious variants (and possibly more virulent variants) appear, because that increased infectiousness gives those variants a competitive advantage. But the immune system counters these new variants by adjusting and improving its innate and naturally acquired immune responses, which then provides sterilizing immunity against these variants and reduces viral infectiousness at the population level. Furthermore, during the course of the pandemic, people’s immune systems, particularly their innate immune systems, become increasingly able to eliminate the virus, due to practice and training (thought to be an epigenetic process). That is why maintenance of an optimally healthy innate immune system is so important. Moreover, during the course of the pandemic, it is also possible that the immune system continually adjusts its responses to the virus, so that those responses are more prompt, more efficient, and less likely to be excessive. The latter is important because hyperimmune responses (e.g. cytokine storm) can be extremely detrimental and life-threatening.
The above interplay between the virus and the immune system at the population level (involving the above factors and other factors) results in the pandemic gradually subsiding over the course of several months (with some expected “waves” occurring during the process). For example, the 1918 influenza epidemic involved 3 waves and subsided after about 10 months.
Until sufficient herd immunity has developed, very careful public health measures are needed to protect the elderly and most vulnerable. It is important to understand that herd immunity via natural infection is far superior to herd immunity attempted via mass vaccination with a suboptimal (non-sterilizing) vaccine in the midst of an active pandemic. Herd immunity cannot be achieved through mass vaccination with a suboptimal (non-sterilizing) vaccine in the midst of a pandemic, and, In fact, such vaccination interferes with development of herd immunity. More on this later.
Treatment of COVID: In the meantime, while the pandemic is following its natural course, it is extremely important to protect infected individuals by providing optimal treatment. Informed physicians know that COVID has two phases—a viral phase and an immune-response phase. [164-181] First, there is a viral phase, which is due to active viral replication and is typically limited to about 7 days, thanks to a normal immune response. In most people the immune response phase is relatively silent; but in some patients, there is a harmful hyperimmune/hyperinflammatory phase that typically starts on about day 8 and causes particularly severe illness during the second and third weeks. This second phase of illness is not due to ongoing viral infection; it is due to an abnormal, dangerously excessive immune reaction to the virus (“cytokine storm,” hypersensitivity reaction, e.g.). [170-176] Fortunately, most people experience only the viral phase (and a relatively silent immune response to the virus) and do not experience a hyperimmune/hyperinflammatory phase. It is the patients in the ICU who are experiencing a severe hyperimmune/hyperinflammatory phase.
In addition to relying on the multi-dimensional response of the immune system to COVID, people who do become symptomatic with COVID may be started on safe outpatient anti-viral therapies (according to their individual needs) as soon as it becomes apparent that they are having symptoms of COVID and are in the viral phase of illness. Such patients may be promptly started on a combination of medications that would be likely to at least somewhat slow viral replication [182-200], plus nutraceuticals to support the immune system (e.g., vit D, vitamin C, Zinc). Those who appear to have an unusually large and/or threatening viral load (which may be estimated by the Ct values at which their serial COVID PCR tests are positive, see Note below), or are otherwise at higher risk, may, in addition, be promptly treated with monoclonal antibodies—unless the involved SARS-C-V-2 variant has become resistant to available monoclonal antibodies. If a patient’s serial studies for d-Dimers become positive, early outpatient treatment with anticoagulation (e.g., oral apixaban) may be initiated. [201-203] In addition, selected patients may be placed on outpatient O2 monitoring with a finger probe, to detect early signs of worsening.
Note: For discussion of Ct (cycle threshold) values of COVID PCR tests, see Section 6 and click on the following link: https://notesfromthesocialclinic.org/the-importance-of-knowing-the-ct-value-at-which-covid-pcr-tests-are-positive-long-version/
The above outpatient treatment efforts, particularly if initiated promptly after onset of symptoms, have been shown to significantly reduce the likelihood of early illness evolving into severe illness that would require hospitalization. [192-193]
If a patient who is receiving the above outpatient treatment shows signs of worsening despite that treatment (on day 8, for example), prompt evaluation for presence of a hyperimmune/hyperinflammatory response may be initiated and the patient may be treated promptly with appropriately aggressive immunosuppression, including corticosteroid and anti-cytokine therapies. [204-223] Antihistamine and anti-leukotriene therapies might also be indicated (though this has not been adequately studied). The need for anticoagulation should also be addressed, both during the viral phase and the hyperimmune phase.
Note: For further discussion of treatment of severe COVID illness, click on the following link: https://notesfromthesocialclinic.org/treatment-of-severe-covid-19-illness-long-version/
With the above prompt outpatient treatment of early illness (the viral phase) and with prompt, appropriately aggressive immunosuppressive treatment of those who develop severe illness due to a hyperimmune reaction, a high percentage of the hospitalizations and deaths that occurred during 2020 and 2021 would likely have been prevented.
The above-mentioned medical treatments, coupled with normal immune function, minimize hospitalizations and deaths. Yes, absolutely, the hospitalizations and deaths that do occur are, of course, regrettable and tragic. However, according to the alternative narrative (as will be explained in the next Section), treatment of the pandemic with a mass vaccination campaign that uses sub-optimal (non-sterilizing) vaccines results in considerably more cumulative hospitalizations and deaths than occurs when such a vaccination campaign is not deployed.
In contrast to the above understanding, Dr. Fauci and the US COVID Task Force believe it is essential to treat a pandemic like COVID with the current COVID mass vaccination campaign, despite the sub-optimal (non-sterilizing) nature of the COVID vaccines. They believe that the current mass vaccination campaign markedly reduces cumulative hospitalizations and deaths. Their belief is that the COVID pandemic has become prolonged and made more dangerous because of unvaccinated people. They contend that unvaccinated people have irresponsibly “allowed” the virus to spread, replicate, and develop new threatening mutations/variants, and they claim that this would not have happened if all had become vaccinated. In their view the initial pandemic has morphed into a “pandemic of the unvaccinated” and this has resulted in many hospitalizations and deaths that could have been prevented if more people had become vaccinated.
As we will see in the next section, those who support the alternative narrative believe that the current mass vaccination campaign, not the unvaccinated population, is responsible for prolonging the COVID pandemic, creating more frequent “waves,” making the pandemic more dangerous, and causing excessive lives to be lost. Those who support the alternative narrative would agree with Dr. Fauci, if the available COVID vaccines were “optimal” (sterilizing) vaccines, capable of eradicating/killing the virus—but the available COVID vaccines are “suboptimal” (non-sterilizing) at best.
SECTION 4: WHAT HAPPENS WHEN A PANDEMIC LIKE THE COVID PANDEMIC IS PRIMARILY TREATED WITH A MASS VACCINATION CAMPAIGN, USING A SUB-OPTIMAL (NON-STERILIZING) VACCINE?
If an optimal (sterilizing) vaccine (meaning the vaccine completely prevents infection and transmission) were available for COVID and were safe, then use of such a vaccine for the most vulnerable (at least) would clearly be helpful and wise. Optimal vaccines kill (fully eradicate) the virus as soon as the virus threatens the individual.
Fortunately, we have had optimal (sterilizing) vaccines for some viruses—e.g., smallpox. These vaccines have typically used either live attenuated virus or dead virus to mimic infection and, thereby, trigger the immune system to launch a multi-dimensional systemic immune response that eradicates the virus if/when it enters our body. These optimal vaccines prevent infection and transmission and have been extremely valuable.
If an optimal vaccine for COVID were available and proven to be safe, the scientists and physicians who support the alternative COVID narrative would fully support its use, at least for the vulnerable. After all, these scientists and physicians are not “anti-Vaxx.” They are pro-vaccination, as long as the vaccines are adequately demonstrated to be safe, effective, and necessary. Many of these scientists and physicians have devoted their careers to development of safe vaccines.
However, historically, virologists and vaccinologists have not been able to develop a safe, optimal vaccine for previous coronaviruses or respiratory syncytial virus (RSV)—viruses that enter the human body through the respiratory tract. [224-231] Over the past 20 years the best they have been able to do is develop a sub-optimal (non-sterilizing) vaccine—meaning that the vaccine might reduce disease severity somewhat but does not fully prevent infection or transmission. Those sub-optimal vaccines have not proven to be adequately effective or adequately safe. In fact, animal studies of these previous coronavirus vaccines (and RSV vaccines) have shown them to be dangerous, primarily because of antibody-dependent enhancement (ADE) of viral replication and disease severity in the vaccinated subjects. [224 -260]
Note: For more detailed discussion of ADE, click on the following link and read the explanation under Question 8 in that document: https://notesfromthesocialclinic.org/a-call-for-an-independent-international-covid-commission/
Despite the above history of coronavirus vaccine failures, the current COVID pandemic (which involves a highly mutable virus) has been primarily managed with roll out of a rapid mass vaccination campaign, across all age groups, using sub-optimal (non-sterilizing) uni-dimensional vaccines (directed at only the spike protein), in the midst of the active pandemic and in the midst of considerable lockdown measures. According to many experienced virologists/vaccinologists (including, most notably, Dr. Geert Vanden Bossche), this type of vaccination campaign is a recipe for failure and harm. [261-270 and LINKS A-I, and O] Why? Because, according to these scientists, the sub-optimal (non-sterilizing) vaccine does not adequately prevent the virus from entering cells, replicating in those cells, and being transmitted to other people. When the virus replicates in the vaccinated person’s cells, new mutations develop. Then, under the pressure of the mass vaccination campaign (and the added pressure of the lockdown measures), the new mutations that will be most successful will be those that are able to “escape” the vaccine-induced anti-spike protein antibody and are also more transmissible. As a consequence, the vaccine-induced antibodies quickly become less effective and the new variant more easily enters cells, more easily replicates, and is more transmissible than its predecessors. Some vaccinologists/pharmaceutical companies might then try to counteract this phenomenon by producing a new, “updated” vaccine against the latest mutated spike protein, but it is impossible to keep up with the new “escape” mutations that inevitably evolve.
In March 2021 Dr. Vanden Bossche dutifully warned the WHO, CDC, and those leading the mass vaccination campaign that this campaign would inevitably and continually lead to the development of variants that would be increasingly transmissible and would become predominant strains. Click on this link to view his warning: https://notesfromthesocialclinic.org/treatment-of-severe-covid-19-illness-long-version/
Dr. Vanden Bossche appears to have been correct. [261-270] Since initiation of the mass vaccination campaign (in December 2020), we have seen one wave after another of new, more transmissible and more vaccine-resistant variants that have achieved predominance, until being displaced by a new predominant variant. The Delta variant accounted for about 97% of COVID infections during its reign and was much more vaccine-resistant than were earlier variants. Now the Omicron variant has become similarly predominant, and it is much more transmissible and much more vaccine-resistant than was Delta. (When pandemics are not treated with mass vaccination, it is unusual to see such total predominance of a single variant; usually there is a mixture of many variants.)
In addition to the concern that the COVID mass vaccination campaign would result in development of predominant variants with increased vaccine resistance and increased transmissibility, Dr. Vanden Bossche has also been concerned that the mass vaccination campaign might eventually generate a predominant variant that is more virulent (deadly) than any of its predecessors—either due to ADE (which primarily affects the vaccinated) or to increased intrinsic virulence of the virus, or both. (See LINK O.) Fortunately, a new variant with increased intrinsic virulence has not yet appeared, but such is certainly possible, particularly if the current mass vaccination campaign is continued. A virus with increased intrinsic virulence would be a threat to both the unvaccinated and vaccinated, including children, particularly if it is more transmissible than previous variants. The extent to which ADE already has, or eventually will, complicate the pandemic is unclear. It is likely that ADE has already caused, or will soon cause, increased disease severity and disease spread, particularly among and by the vaccinated, and particularly if the mass vaccination campaign is continued.
Dr. Vanden Bossche is also concerned that the COVID vaccines may be undermining and disrupting our normal immune system—particularly our innate immunity, particularly in children. For example, he is concerned that the vaccinal antibodies, which tightly bind to the spike protein of the SARS-CoV-2 virus, “out compete” our immune system’s natural antibodies (rendering the innate immune system to become less functional) and may be otherwise disrupting the natural flow and natural training of our immune system. He appreciates the complexity and delicacy of the immune system (our immune ecosystem) and fears that the vaccines are harmfully tampering with our immune ecosystem, rendering it (the immune system) less capable of protecting us.
Dr. Vanden Bossche (and other scientists who support the alternative narrative) disagrees that this is a “pandemic of the unvaccinated.” On the contrary, he views it as a pandemic that has become prolonged and more dangerous because of the mass vaccination campaign with its suboptimal (non-sterilizing) vaccines. Furthermore, he worries that it is the vaccinated people who are becoming the most likely “spreaders” of the virus, for the following reasons: vaccinees “selectively” transmit the more infectious immune escape variants, which leads to “enrichment” of these variants in the viral population when the virus circulates in a highly vaccinated population; vaccinees continuously serve as a breeding ground for more infectious variants because vaccinal antibodies cannot mediate/ facilitate killing of virus-infected cells; and, because the vaccine might indirectly make the vaccinee somewhat less symptomatic (when subsequently infected), even asymptomatic, the vaccinee may become an unwitting asymptomatic “super-spreader.” If this is true (which is highly likely), then it is the vaccinated people, not the unvaccinated, who are the greater threat to the vulnerable, to the elderly, and to children. He also worries that, because of ADE, the vaccinated may well experience more severe disease than the unvaccinated.
Dr. Vanden Bossche, who is one of the most experienced and wisest vaccinologists in the world, thinks it is a huge mistake to continue the current COVID mass vaccination campaign. (See LINKS A-I and, particularly LINK O, his most recent article.) He argues that now, with the Omicron variant being relatively mild and being the predominant variant, is the best time to stop the vaccination campaign—because the Omicron variant, though very transmissible, is fortunately causing less severe disease than any of the preceding variants, and cessation of the mass vaccination campaign would allow some restoration of normal healthy innate immune function. Additional vaccination would only further boost (useless) vaccinal antibodies and further compromise functionality of relevant innate antibodies. He suggests that we stop vaccinating before it is too late—before an even more transmissible and more virulent variant is generated (by mass vaccination), before ADE becomes more common, and before the immune systems of a higher and higher percentage of the population become compromised/hampered by the vaccines. He emphasizes that the greatest contributors to herd immunity are those who are unvaccinated and develop naturally acquired sterilizing immunity. If everyone becomes vaccinated, there will be no one left to develop robust, unfettered naturally acquired sterilizing immunity—and, therefore, further development of herd immunity ceases. His admonition includes a plea to not start vaccinating people with a new vaccine that is directed against Omicron’s mutated spike protein. Such a vaccine would be only transiently beneficial, if at all, and would soon become obsolete when an inevitable new vaccine-resistant strain appears. Furthermore, continued vaccination with a new, updated vaccine will further delay restoration of natural immunity that he fears has already been disrupted and harmed by COVID vaccines. And continued vaccination will further delay development of herd immunity.
The worst possible scenario (caused by continued mass vaccination) would be the development of an extremely transmissible, more intrinsically virulent, vaccine-resistant strain that a fully vaccinated and ADE-prone population is unable to handle and that even unvaccinated children’s immune systems might have difficulty controlling. This would be a disaster. In such a situation we would need a well-coordinated mass anti-viral treatment campaign (not a mass vaccination campaign), and we would be even more dependent on improving people’s general health (with proper exercise and nutrition and avoidance of obesity, etc.), while maximizing use of early outpatient treatment and optimally treating inpatients.
According to the alternative narrative, the total cumulative numbers of COVID hospitalizations, COVID ICU admissions, and COVID deaths during the COVID pandemic (from the beginning of the pandemic through January 2022) would have been lower if the pandemic had not been treated with the mass vaccination campaign and, instead, had been treated as described in Section 3.
For further discussion, details, and references, regarding the concerns of Dr. Vanden Bossche and those who agree with him, please see the LINKS to videos at the end of this Open Letter and/or click on his website: https://www.voiceforscienceandsolidarity.org/
So, which narrative is correct? Who is correct? The US COVID Task Force and the CDC? Or Dr. Vanden Bossche? Whose understanding of immunology, virology, vaccinology, epidemiology, and evolutionary biology is deeper, more thoughtful, and more accurate? Whose understanding is more careful and compassionate? How is a parent to know? How is their pediatrician to know?
It would be wise and helpful to assemble an inclusive and diverse expert panel of fairly selected, internationally respected virologists and vaccinologists to carefully, critically, respectfully, transparently, and publicly examine the two contradictory narratives regarding COVID vaccination. Indeed, Dr. Vanden Bossche, in an open letter to the global medical community, pleaded (12 months ago) for just that. Such dialogue and careful examination is fundamental to Medicine and Science but has been largely absent during the COVID pandemic. Instead, we have seen intolerance of challenges to the prevailing narrative. Dr. Vanden Bossche’s plea for healthy scientific dialogue has gone unheeded, so far. Instead, scientists who support the alternative narrative have been censored and demonized as deplorable “spreaders of harmful disinformation.”
SECTION 5: OTHER CONCERNS ABOUT THE COVID VACCINES—ADVERSE EVENTS:
In addition to Dr. Vanden Bossche’s concerns that current mass vaccination is driving the development of more transmissible and potentially more lethal strains; is harming natural immune function; is delaying development of naturally acquired herd immunity; and threatens to cause more severe COVID illness and increased numbers of COVID deaths; many scientists and physicians are deeply concerned that the COVID vaccines are unsafe in other important ways—causing unacceptable short- and long-term side effects for individuals (e.g., myocarditis in adolescents and young adults; and death from blood clots in adults). [271-1028]
Please note that in the REFERENCES section of this Letter I have listed 757 articles on serious adverse effects of the COVID vaccines. [271-1028] Almost all of these articles represent peer-reviewed publications in the formal medical literature; the remaining are pre-prints of articles that have been submitted for such publication. This represents an alarming and unprecedented number of published reports about the side effects of a new pharmaceutical product—and barely a year has passed since roll-out of the COVID vaccines. I urge the reader to at least scroll through the list of REFERENCES and read the titles of the 757 articles. These articles do not represent “misinformation.”
The two available mRNA vaccines (Pfizer and Moderna) are lipid-nanoparticle-formulated, nucleoside-modified mRNA vaccines that encode the spike glycoprotein of the SARS-CoV-2 virus. [882]
The mRNA vaccines work by entering human cells and directing them to produce the spike protein. Once those cells produce the spike protein, the spike protein ends up sitting on the outside of the cell wall, where it is recognized by the immune system as an unwanted and potentially dangerous foreign substance. The immune system then produces antibodies against the spike protein. To an unknown extent, some of the spike protein breaks free and circulates through the bloodstream, before it becomes neutralized by the vaccinal antibody. Although high levels of anti-spike antibody have been demonstrated in vaccinees (at least for several weeks or a few months), there has been no evidence that any of the COVID vaccines (including the genetic vaccines) induce spike-specific cytotoxic memory cells.
Of considerable concern is the possibility that, when a vaccinated person becomes infected with SARS-CoV-2, the attachment of vaccinal antibody to the spike protein expressed on the surface of the transfected cells could lead to ‘trans’ fusion and, therefore, formation of syncytia. (See LINK O.) The latter are known to correlate with deeper penetration of infection, resultant organ damage, and severe inflammatory disease—i.e., more severe disease in the vaccinee, because of their vaccination.
Historically, during the development of mRNA gene therapy technology, two major problems became apparent—unmodified mRNA is too immunogenic (stimulates the immune system too much) and too unstable to be clinically feasible [882, 1029]:
Unmodified mRNA proved to provoke a degree of immune reaction that rendered it dangerous and unfeasible for clinical use. [1029] For example, by stimulating Toll-receptors, unmodified mRNA adversely activates cells of the innate immune system and increases production of type 1 interferon and pro-inflammatory cytokines. It became necessary to modify the mRNA so that it would be less immunogenic. This was accomplished by incorporating pseudouridine into the mRNA. This nucleoside-modified mRNA proved to not only suppress unwanted mRNA-mediated immune activation, but also enhance the stability and functionality of the mRNA.
A potential problem with nucleoside-modified mRNA is that it is less immunogenic because it dampens innate immune sensing. This suggests that it might at least transiently suppress innate immunity when injected into humans. This, in turn, raises the possibility that some people who receive the vaccine may develop at least a transient and possibly clinically significant decrease in innate immunity functionality shortly after vaccination. Indeed, in a phase I/II study of mRNA COVID-19 vaccine, healthy volunteers (under age 56) developed a transient, dose-dependent lymphopenia during the first few days after initial vaccination. [1030] Whether interferon and cytokine levels also dropped is unclear, because this was apparently not studied (or at least not reported). These data raise the possibility that some elderly people, who may already be relatively less immunocompetent (because of age), may not tolerate this transient immunosuppression as well as younger, healthier people—i.e., the immunosuppression may be less transient and/or more clinically significant. It is conceivable that this could predispose some elderly people to develop a post-vaccination infection (by whatever infectious agents happen to be around).
So, one concern about the mRNA vaccines is that these vaccines might cause a transient immunosuppression, during the first 1-4 weeks after the initial dose of vaccine, that could render elderly people transiently more vulnerable to becoming infected (with whatever bacteria or virus they may be exposed to, including but not limited to COVID). It is conceivable, for example, that an elderly nursing home resident who suddenly and inexplicably deteriorates and dies during the weeks after COVID vaccination might do so because transient vaccine-induced immunosuppression has made the resident more vulnerable to infection. This possibility needs to be at least kept in mind. After all, alert innate immune sensing, type 1 interferon, cytokines, and lymphocytes play major roles in our initial innate immune defense against infection.
Although eventual studies may prove that there is little or no need to be concerned about the above, this issue has not yet been adequately studied.
A second problem with the mRNA technology is that unmodified mRNA is normally quickly degraded, before it has a chance to instruct the ribosomes in our cells to synthesize the desired protein (spike protein in the case of the COVID vaccine). [882, 1029] So, to make the technology clinically feasible, the mRNA must be protected from degradation. This is done by encapsulating the mRNA in a lipid-nanoparticle. The lipid is polyethylene glycol (PEG). This lipid encapsulation protects the mRNA from degradation, makes it more stable, and enhances its entry into the cytoplasm of cells. [1029]
This raises the following unanswered question: Since it is protected, how long does this PEG-encapsulated mRNA remain in our cell’s cytoplasm before it eventually disintegrates? Does it persist for just hours, or for days? weeks? months? Do we know? This is important, because if the mRNA continues to instruct the cell’s ribosomes to make spike protein only for a matter of hours or days, this might be reasonable. But if the PEG-encapsulated mRNA persistently instructs the ribosomes to keep making spike protein for weeks and weeks, even months, this might create prolonged and harmful (not to mention unnecessary) immune reactions to this prolonged production of spike protein. [786, 882] In this way could the vaccine be predisposing some people to chronic unnecessary and potentially harmful immune reactions?
This raises a third concern. We want a vaccine to provoke an appropriate immune reaction for an appropriate length of time. We do not want to provoke an excessive reaction or a reaction that persists for an excessive length of time. It is unclear whether the mRNA vaccines provoke only an appropriate reaction (i.e., an appropriate level of antibody production, not too high or too low) and only for an appropriate length of time. Although eventual studies may prove that there is little or no need to be concerned about the above, this issue has not yet been adequately studied.
A fourth concern: How healthy is it to have PEG-encapsulated mRNA sitting in the cytoplasm of our cells? Do we know with certainty which cells of the body receive the vaccine’s PEG-encapsulated mRNA? Is it just the muscle cells near the site of injection? Does the PEG-encased mRNA enter all cells that have a lipid cell membrane, including brain cells, heart cells, ovarian cells? [882] Do we know the consequences of housing PEG-encapsulated mRNA in our various cell types?
According to biodistribution studies in rodents, conducted by Pfizer, the mRNA (or at least the lipid nanoparticle) does not just stay in the arm muscle that has been jabbed. It gets into the regional lymph nodes, then the general circulation, and enters the cells of many organs, including the liver, spleen, other lymph nodes, adrenal glands, ovaries and testes, and probably the heart and brain. [882, 1031] These studies were of sub-optimal quality and will need to be repeated but are very concerning and are consistent with what is known about intramuscular injections in general. It is not normal, healthy, or wise for cells in these organs to be producing spike protein.
As already mentioned, we do not know how long the mRNA continues to make the cells synthesize the spike protein. Does this vary from person to person and from organ to organ? We also do not know how much spike protein is produced in a given person, or within various organs of a given person. This quantity might vary from person to person and within organs of an individual person, and it undoubtedly varies over time. Some people and/or some organs may produce much larger quantities of spike protein than others.
We also do not know how exposure to spike protein after vaccination compares to exposure to spike protein during actual COVID infection. The acute viral phase of the infection typically lasts approximately one week, during which time the spike protein in the infected body reaches a peak, then most likely declines relatively quickly and greatly, then disappears. It is possible that COVID vaccination results in a much greater and/or longer exposure to spike protein—higher levels, longer levels, or both. This is unknown.
Nor do we know how much anti-spike antibody is produced (after vaccination) by a given person and for how long. This might vary from person to person and within organs of an individual person. Dr. Vanden Bossche is concerned about: 1) the potential for “trans” fusion between cells that express spike protein and normal target cells; and 2) relatively mild but prolonged immune stimulation. This could lead to internalization of detached spike protein into dendritic (antigen-presenting) cells. Prolonged production of spike protein could therefore lead to prolonged antigen presentation and, therefore, prolonged/ chronic immune stimulation of CD4+ T helper cells. This would typically predispose to immune inflammatory disease, including autoimmune disorders. A further concern is that we do not know the extent to which a given person’s immune system might mount a cytotoxic attack against the spike protein expressing cells (after vaccination), and, if so, for how long.
As just one example, if a particular young, vaccinated woman’s ovaries start making huge quantities of spike protein (compared to quantities produced by other vaccinated women) and her immune system mounts a particularly vigorous anti-spike protein antibody attack and/or a particularly vigorous and/or prolonged cytotoxic attack on spike protein-coated ovarian cells, that woman would be at risk of diminished fertility. This issue has not been adequately studied.
A fifth concern: A significant percentage of people are allergic to PEG. Anti-PEG antibodies have been found in 42-72% of people. [882] Some such people may have allergic reactions, including potential life-threatening anaphylaxis and prolonged anaphylaxis. [427, 487, 619, 640, 641, 669, 678, 691, 732, 822, 844, 848, 881, 882, 960, 999] How do we know who might have an initial, prolonged, or delayed allergic reaction to the PEG component of the vaccine?
A sixth concern: Are the mRNA vaccines setting people up for either transient or chronic autoimmune reactions? An example of this concern might be the Florida obstetrician who died of autoimmune thrombocytopenia shortly after receiving a mRNA vaccine. [1032] Shoenfeld et al have emphasized the potential capacity of the SARS-CoV-2 virus to trigger a variety of autoimmune reactions, including reactions occurring due to “molecular mimicry.” [1033-1035] “A massive heptapeptide sharing exists between the SARS-CoV-2 spike glycoprotein and human proteins.” [1034] There is a legitimate concern that the antibodies to spike protein that the mRNA vaccines train the immune system to produce might accidentally cross-react with peptides within normal human tissue (the phenomenon of molecular mimicry), either transiently, recurrently, or chronically. Although future studies might prove that the mRNA vaccines do not trigger significant molecular mimicry reactions, or other autoimmune reactions, this issue has not yet been adequately studied.
A seventh concern is the possibility that antibody dependent enhancement (ADE), or other similarly violent immune reactions, might occur in vaccinated people when, at a later date (many months, even years, later), they become infected with the wild SARS-CoV-2 virus. [224-260, 882] For more information about ADE see Question 8 in this link: https://notesfromthesocialclinic.org/a-call-for-an-independent-international-covid-commission/
An eighth concern is that the spike protein all by itself appears to have potential to do great harm. [354, 682, 780, 847, 882] This is true when the spike protein is present during active COVID illness, or when the spike protein is produced solely as a result of vaccination. Spike protein appears to be a toxic, pathogenic (harmful) material. It can cause damage not only in people with active COVID illness, but also in vaccinated individuals (i.e., in the absence of infection). The spike protein can abnormally activate platelets, which predisposes to thrombosis (clotting). [525, 555, 702, 1026] The spike protein can also injure the endothelial cells that line the inner walls of blood vessels, which predisposes to thrombotic occlusion and/or conceivable microvascular occlusion from endothelial cell swelling. [682, 780] The spike protein can cross the blood-brain barrier and potentially injure the brain. [354, 847] The spike protein can lead to lung injury [332] and myocarditis/pericarditis [391, 448, 563, 740, 811, ]. And the spike protein can injure the liver, causing immune-mediated hepatitis. [349, 841, 851] Again, this injury can occur due to vaccination alone, in the absence of ever being infected with the SARS-CoV-2 virus.
Myocarditis/pericarditis after COVID vaccination: The potential for COVID vaccination to cause worrisome myocarditis/pericarditis deserves special attention, because of its frequency and because young adolescent males are the most prone to develop this complication. Of the 757 articles listed in the REFERENCES, 157 are about myocarditis and other serious cardiac complications occurring after COVID vaccination. This represents an unprecedented number of published reports about cardiac side effects of a new pharmaceutical product—and barely a year has passed since roll-out of the COVID vaccines. In addition, as of July 2021 there had been 1226 cases of post-COVID vaccination myocarditis reported in VAERS (Vaccine Adverse Effects Reporting System), including 627 cases of myocarditis in persons under 30 years of age.[448] (VAERS data will be discussed further, later.)
Vaccine-induced myocarditis occurs most frequently in males under 40 years of age [391, 448, 563, 811], particularly in 12–15-year-old boys [563], particularly after the second dose of vaccine [811], and particularly with the Moderna vaccine [811]. Chua, et al reported an incidence of myocarditis in adolescent males after COVID vaccination of 1 in 2679 vaccinated persons (37.32 cases per 100,000 persons vaccinated.) [391] Patone et al have reported that in young males (less than 40 years of age) the risk of developing myocarditis after COVID vaccination is greater than the risk of developing myocarditis after having a positive COVID test. [811] In a study of 16–19-year-old males, Mevorach et al reported that the risk of developing myocarditis was 8.96 greater in vaccinated individuals, compared to unvaccinated, and was 13.6 times greater in vaccinated, compared to historical controls. [740] VAERS data suggest that the COVID vaccines generate cases of myocarditis/pericarditis at a rate that is 860 times greater than the rate at which typical flu vaccines generate myocarditis/pericarditis. [1036]
Neurologic complications after COVID vaccination: Another large category of severe adverse events after COVID vaccination is neurologic disease—strokes, brain hemorrhage, Guillain-Barre, etc. Of the 757 articles listed in the REFERENCES, 175 are reports of serious neurologic consequences. This represents an unprecedented number of published reports about neurological side effects of a new pharmaceutical product—and barely a year has passed since roll-out of the COVID vaccines. It is unclear whether COVID vaccines have the potential to cause prion disease, but this possibility would be devastating, needs to be taken seriously, and warrants further careful study. [416, 882, 1037, 1038]
Clotting abnormalities after COVID vaccination: Another similarly large category of side effects is abnormal clotting—thrombosis [318, 322, 330, 340, 348], platelet dysfunction [319, 334], and hemorrhage (bleeding) [335, 336]. Of the 757 articles in the REFERENCES, many are reports of life-threatening clotting abnormalities, including devastating clots in large vessels in the brain [340, 348] and gastrointestinal tract [318, 322].
Increased risk of infection and cancer (?) due to T-cell depletion after COVID vaccination: A more recent concern is that mRNA vaccines might cause clinically significant T-cell depletion. One hypothesis is that vaccinal antibodies outcompete innate natural antibodies; this suppresses innate immunity and, thereby hampers virus clearance by NK cells; which then may lead to enhanced activation and hence, exhaustive depletion of CD8+ T cells (cytolytic T cells). Another hypothesis is that mRNA vaccines could conceivably enter immune cells, e.g., lymphocytes, and direct those cells to synthesize spike protein, which is then expressed on the surface of those lymphocytes. Then, natural killer cells (of the innate immune system) and/or cytolytic T cells (of the acquired immune system) might attack and kill the spike-synthesizing lymphocyte—causing a considerable depletion of our immune system’s precious lymphocytes. [1039] We need normal quantities and function of lymphocytes to fight off acute infections, prevent the unleashing of dormant infections (like TB and herpes zoster), and to protect us from developing malignancy (cancer). [882, 1039] If the vaccines are causing either exhaustive depletion or cytolytic destruction of our lymphocytes, this makes us immunodeficient and predisposes us to infection and to cancer. [1039]
Autopsies performed on patients who have died after receiving COVID vaccination: Burkhardt has thoroughly studied the pathology (light microscopy and immunohistochemistry) in 15 autopsies of people who died after COVID vaccination. [1040] In 12 of the 15 cases, it was concluded that COVID vaccination was either “very likely” (5 cases) or “likely” (7) to have been the cause of death. In 2 cases COVID vaccination was considered to be a “possible” cause of death. In 1 case there was no connection between the death and vaccination. The detailed autopsies revealed presence of impressive and extensive inflammation (lymphocytic infiltrates) in various organs, consistent with immune-mediated adverse reactions. It was concluded that severe, organ-threatening and life-threatening inflammatory reactions can be triggered by the COVID vaccines. There is great need for more postmortem examinations of people who have died after COVID vaccination. [874, 885, 924]
On the other hand, other published studies state that the COVID vaccines are quite safe. [320] Dr. Fauci, the CDC, the US COVID Task Force, and the conventional media have confidently and repeatedly reassured the public that these vaccines are safe. They have rarely mentioned any side effect issues, and then usually dismissively, saying that worrisome side effects are “very rare.” The impression is given that the benefits of the vaccines clearly and greatly outweigh any risks. The above-mentioned 757 referenced publications on serious adverse vaccine-related side effects and the worrisome VAERS data discussed below, suggest that the risks of COVID vaccination outweigh the benefits.
VAERS Data: Unfortunately, the CDC/NIH has not been adequately documenting and studying adverse reactions to the COVID vaccines. One would think that part of the massive vaccination campaign, in which a potentially dangerous inadequately tested experimental vaccine is being given to billions of people, would include a compulsive, thorough, efficient, scientifically sound, and mandatory monitoring system to document the extent and nature of adverse reactions. Though promised, no such system has been implemented. Instead, only a voluntary, very cumbersome, inefficient system has been used—the Vaccine Adverse Events Reporting System (VAERS). This system captures only a minority of adverse events. [1036, 1041, 1043] According to analysis by Pantazatos, et al, VAERS deaths are underreported by a factor of 20. [1041] According to analysis by Rose, et al, VAERS data are underreported by a factor of 41. [1036]
Even with its gross inadequacies and underreporting, the VAERS database has revealed a disturbing number of worrisome complications and deaths. [1036, 1041, 1042, 1046] An analysis of cumulative US VAERS data from December 2020 through January 7, 2022, has revealed the following, in the USA alone: [ 1042]
- Total adverse reactions: 723,042
- Life-threatening events: 11, 228
- Deaths: 9,936
- Hospitalizations: 47,837
- Permanent disabilities: 11,625
Taking underreporting into account, it is possible that the above numbers might be 20-41 x higher in reality. [1036, 1041]
For perspective, from 1990-2021 (30 years) VAERS received 5,241 total reports of death due to all non-COVID vaccines, combined, over the course of 30 years. [1042] The risk of death from the COVID vaccines has been reported to be 1 in 29,777, which is 170 times greater than the risk of death from flu vaccines. [1042]
Taking into account that VAERS data may be underreported by a factor of 20-41, Rose estimates, conservatively, that at least 150,000 people in the US have died from COVID vaccination, over the course of one year. [1036]
Further analysis of VAERS data by Rose, suggests that the annual number of reported serious adverse events associated with the COVID vaccines has been multiple times greater than the annual number of reported serious adverse events associated with all non-COVID vaccines, combined. [1036] For example:
- Strokes: 326 x greater with COVID vaccines than with all other vaccines, combined
- Pulmonary embolism: 328 x greater with COVID vaccines
- Deep vein thrombosis: 264 x greater
- Other Thromboses: 250 x greater
- Death: 58 x greater
- Cardiac arrest: 75 x greater
- Myocarditis: 43 x greater
- Intracranial hemorrhage: 42 x greater
Children have died after receiving COVID vaccination. According to VAERS data, during the 6 months prior to July 30, 2021, 14 children and adolescents in the 12-17 age range died after receiving COVID vaccination. [1046] If these deaths were truly due to COVID vaccination, and if deaths in the VAERS database are underreported by a factor of 20-41 [1041, 1036], then the actual number of deaths in the 12–17-year-old age range might be as high as 280-574, over the course of just 6-7 months.
For perspective, it is rare for a previously healthy child to die from COVID itself.
- During the first 17 months of the COVID pandemic in Japan (March 2020-September 2021), no Japanese child died from COVID, according to the Japanese Ministry of Health, Labor, and Welfare. [1047]
- During the first 15 months of the pandemic in Germany, no healthy German children between the ages of 5-12 died of COVID. [1048]
- During the first 4 months of the pandemic in Sweden, none of Sweden’s 1,951,905 children died of COVID. [1049]
- In England, during the first 12 months of the pandemic, 25 children and young people (CYP) died of COVID, out of a total population of 12,023,568 CYP, which translates to 1 COVID death per 480,000 CYP. [1050]
- In the above English study 99.995% of CYP with a positive COVID test survived.
- In the USA, during the first 18 months of the COVID pandemic, 470 children died of COVID in the USA. [1051] For comparison, during the 5-month duration 2017-18 seasonal influenza epidemic (which was a flu season that most of the US public does not remember), 641 children died of influenza (in the USA) [1051]
So, which is the greater threat to children and adolescents—the COVID vaccine or the COVID illness? Bear in mind that COVID is an acute illness that is only rarely severe in previously healthy children and adolescents; and for severe COVID we have excellent treatments that, if used promptly and appropriately aggressively and wisely, can probably prevent almost all of the deaths and other worrisome outcomes that have occurred in children and adolescents. We can successfully control COVID, even very severe COVID, especially in children, if we treat it properly.
In contrast, we have much less control over the adverse events associated with COVID vaccines. In addition to worrisome short-term complications of the COVID vaccines, there are worrisome potential long-term complications that could persist for a lifetime and adversely affect not only individual persons, but humanity as a whole. [882] These long-term complications are of concern even in those who have noticed no problems after their initial COVID vaccinations, and they will likely become more common after more booster shots are given.
As a pediatrician, pediatric rheumatologist, and a grandfather, I worry more about the consequences of the COVID vaccines than I do about COVID itself. But, as I have stated throughout this Open Letter, it would be best to assemble an Inclusive Independent International COVID Commission to address this issue and the other issues raised in this Letter. So far, that has not happened.
Unfortunately, the CDC and US COVID Task Force have been far behind in investigating and examining the data that have been reported to VAERS. Despite the above VAERS data, the COVID Task Force and the mainstream media have continued to barely mention these adverse events to the public (other than to mention “very rare” clots and “very rare” myocarditis) and have continued to confidently reassure the public that the vaccines are “safe.” Adverse reactions have been largely dismissed because they are “very rare” and “the benefits of the vaccine greatly outweigh the risks.” But these statements do not seem to be true?
If the SARS-CoV-2 virus were as deadly as some initially feared (as deadly as SARS-1, MERS or Ebola virus), then the risks of vaccination, even if considerable, might be worth taking. But given the fact that the infection fatality rate (IFR) of COVID has been comparable to that of recent influenza viruses of above average severity (see Lead Article, Question 1 for discussion of the IFR of COVID: https://notesfromthesocialclinic.org/a-call-for-an-independent-international-covid-commission/); given the fact that healthy individuals under 50 (particularly children) are at very low risk of dying or being severely harmed by COVID; given the fact that excellent treatments are available for COVID (including treatment of those who head into severe illness); given the genius and experience of the human immune system; given that the vaccines might be disrupting our immune ecosystem and harming children’s innate immunity; given the worrisome potential and actual side effects of the vaccines and their role in driving the predominance of new worrisome variants of concern; given the mounting evidence of vaccine failure (see Section 7), and given all the other unknowns; the risks of the COVID vaccines appear to outweigh the modest (at best) benefits—particularly for children.
Out of an abundance of caution, it would seem wise to halt the vaccination campaign until adequate study of safety (and efficacy) has been done.
Who is correct? Are these COVID vaccines “quite safe,” or “quite dangerous?” Are they causing greater good than harm, or more harm than good? [882, 1053]
As with other controversial issues discussed in this Letter, it would be wise to assemble an inclusive and diverse panel of fairly selected, internationally respected virologists and vaccinologists to carefully, critically, respectfully, and publicly examine this issue—with all proceedings being televised in a CSPAN-type of format. As part of informed consent, the public certainly deserves to know and needs to know the full spectrum of side effects of these vaccines, including death, and the likelihood of their occurrence.
SECTION 6: PROBLEMS WITH THE COVID PCR TEST AND COVID DATA COLLECTED TO DATE:
Throughout the COVID pandemic, data regarding the number of COVID cases, number of COVID hospitalizations, and number of COVID deaths have been based, fundamentally, on the COVID PCR test. Unfortunately, the COVID PCR test has been used in a scientifically unsound and misleading fashion:
In order to accurately interpret a positive COVID PCR test, the Ct (cycle threshold) value at which the test is positive must be known. The Ct value is an indirect, but very helpful, indicator of how strongly positive the result is. If the test is positive at a low Ct value (e.g., 20), this means the specimen has a large amount of virus in it, the test is strongly positive, and the person is highly contagious. If the test is positive at a Ct value of 40, or 45, or higher, the result is only very weakly positive and is either falsely positive or, at best, the test is detecting just a few remnants of dead virus. A study by Tartof, et al, [1052] reveals (in the subtle details) that when a COVID PCR test is positive at a Ct value greater than 27, the false positivity rate for the test is 75.5%—(349 +1452)/465 +1918) = 1801/2383 = 75.5%. The higher the Ct value, the more likely that a positive test result is a false positive.
Note: For a detailed explanation of Ct values, including many references, click on the following link: https://notesfromthesocialclinic.org/the-importance-of-knowing-the-ct-value-at-which-covid-pcr-tests-are-positive-long-version/
Unfortunately, the Ct value at which a COVID PCR test is positive has not been routinely disclosed, except in some research settings. Instead, patients and their physicians have been routinely told only that the test is positive or negative, with no information about the Ct value when the test is positive. Most COVID PCR test kits are programed to report a positive result if the test is “positive” at a Ct of 45 or lower—but the actual Ct value of a positive result is not included in the printout of the test result and is not available to the physician or patient, even if they think to ask for the Ct value at which the test was positive. The printout indicates only “positive” or “negative,” with no mention of Ct values. Most patients and physicians have not known the importance of asking for the Ct value at which the test was positive.
Most knowledgeable lab experts would agree that the COVID PCR test kits should report a result as positive only if it is positive at a Ct less than 28 (or less than 30, at most)—because a positive result at a Ct greater than 28 (or 30) is most likely a false positive, or at least inadequately interpretable, and, therefore, misleading.
Furthermore, even when a COVID PCR test is positive at a Ct less than 28 (meaning that it is a true positive), it is unclear whether such a result is truly always specific for SARS-CoV-2. Despite the manufacturers’ claims that their tests are highly specific for SARS-CoV-2, there is legitimate concern that other viruses (besides SARS-CoV-2) might cause a COVID PCR test to be positive (even at a low Ct value). Adequate, independent determination of the true specificity of commercially available COVID PCR tests has not been done.
The only way to determine with certainty whether a person has COVID is to perform genomic sequencing of a person’s specimen—preferably, Sanger genomic sequencing. [1077] Since the beginning of the pandemic it has been feasible (scientifically, logistically, and financially) to insist that all COVID testing (throughout the USA, for example) be done via genomic sequencing of specimens. Unfortunately, genomic sequencing tests have not been routinely performed or even made routinely available. To date, genomic sequencing of specimens has only been done in certain research settings, including periodic testing by the CDC to determine variants of concern.
As the above discussion reveals, the COVID PCR test has been used, throughout the pandemic, in a way that has generated inadequately interpretable, frequently inaccurate, and, therefore, misleading results. Inaccurate, misleadingly high numbers of positive COVID PCR tests have resulted.
The data regarding numbers of COVID cases, COVID hospitalizations, and COVID deaths have also been of low scientific quality and misleading—both because of the scientifically unsound way in which the COVID PCR tests have been used and because scientifically unsound criteria have been used for designation of COVID cases, COVID hospitalizations, and COVID deaths. For example, a “COVID case” has been anyone who has a positive COVID PCR test (even at a Ct of 45), even if the person is asymptomatic, has had no contact with anyone who has had COVID, and was tested simply for screening purposes. It is scientifically unsound to designate such a person as a “COVID case.”
A “COVID hospitalization” has been anyone who, while in the hospital, was noted to have a “positive COVID test,” even if the patient had no symptoms of COVID, no contact with anyone with COVID, was admitted for a non-COVID reason, and was simply tested on admission (or during their hospitalization) for screening purposes. Such a patient probably (i.e., statistically) does not have COVID. The only way to know with certainty would be to do genomic sequencing, which is not routinely done. It is scientifically unsound to designate such a person as a “COVID hospitalization.”
A “COVID death” has been anyone who died and was noted to have had a “positive COVID test” at some point during the 28 days prior to death. Throughout the pandemic there has been little or no effort to carefully distinguish between “death from COVID” (death definitely due to COVID) and “death with COVID” (death with a coincidental positive COVID test, but the death was not due to COVID). It is scientifically unsound to designate a death as a “COVID death” if they clearly died of something else and just coincidentally had a “positive COVID test,” particularly if the test was “positive” at a Ct greater than 30.
The scientifically unsound use of COVID PCR tests and the closely related scientifically unsound criteria used for designation of “COVID cases,” “COVID hospitalizations,” and “COVID deaths,” has resulted in US data (e.g., CDC data and state health department data) being scientifically unsound, of unacceptably low scientific quality, inadequately interpretable, inaccurate, and misleading. This has made it impossible to know with certainty how many true COVID cases, COVID hospitalizations, and COVID deaths have occurred during the course of the pandemic. This is not the way science and medicine are meant to be practiced. Fundamental principles of careful scientific data collection have been violated. It is not too late to begin collecting COVID data in a proper, scientifically sound fashion.
Some might argue that the decision to employ excessively loose criteria for a positive COVID PCR test and loose criteria for designation as a COVID case, COVID hospitalization, and COVID death represented an innocent effort to “cast a wide net and not miss any cases, out of an abundance of caution,” especially during the early weeks of the pandemic when little was known about COVID. But in that case the public should have been fully informed (22 months ago) of the importance of knowing the Ct value at which a PCR test was positive and that the reported numbers of COVID cases, hospitalizations and deaths might be misleadingly high, because of the loose criteria being used. And these criteria should have been tightened within a couple of months, after it should have been obvious that they were much too loose and were interfering with meaningful data collection. But the public (and even physicians) were not informed, and use of these criteria and misuse of the COVID PCR test have been continued for the past 22 months, resulting in erroneous data and unnecessarily high levels of fear, anxiety, social frustration, and financial hardship.
The decision to use these criteria and not fully inform the public (particularly about the Ct values) was certainly an error in judgment; and it could be argued that it represented medical and scientific malpractice. Some might argue that it has represented medical and governmental malfeasance. In defense of decisions made, some might argue that provision of Ct values “would have been too complicated for the public to understand,” but that is both untrue and insulting to the public. The public is quite capable of understanding what this letter has explained about Ct values.
The above problems with data collection are obviously relevant to initial and subsequent studies of vaccine efficacy, because many of those studies have primarily been based on scientifically unsound use of the COVID PCR test and use of scientifically unsound criteria for designation of “COVID cases,” “COVID hospitalizations,” and “COVID deaths. Studies of COVID, including vaccine efficacy studies, which are based on scientifically unsound use of the main diagnostic test (the COVID PCR test) and scientifically unsound criteria (for “COVID cases,” e.g.) will, obviously, yield scientifically unsound results and must be interpreted with caution. That is why it is so important to practice science, medicine, and research with impeccable scientific rigor.
Further complicating reports of vaccine efficacy has been the variability, complexity, and lack of clarity, regarding the definitions of “unvaccinated” and “vaccinated” used in a given study—not to mention lack of accuracy in the categorization of individual people.
SECTION 7: EFFICACY OF THE COVID VACCINES:
How effective were the COVID vaccines in the beginning? How effective are they now?
For the following three reasons it was predictable that the COVID vaccines might prove to be less effective than initially thought.
First, recall that in Section 2 we talked about the two compartments of the immune system—the mucosal Immune System (the “Air Force”) and the Systemic Immune System (the “Navy”). And we pointed out that the currently available COVID vaccines provide uni-dimensional training of the systemic immune system, but little if any training of the mucosal immune system—which is a problem because the SARS-CoV-2 virus enters the body through the respiratory tract and only later threatens to invade the systemic compartment. It would be much better to have a vaccine that provides multi-dimensional training of the entire mucosal immune system and the entire systemic immune system. Historically, however, it has been very difficult to develop a safe vaccine of that type. Prior attempts have not only failed but caused great harm. (See Section 4.)
Second, recall that in Section 4 we explained that the currently available COVID vaccines are sub-optimal. They do not fully prevent entry of virus into human cells. Unlike optimal vaccines, they do not prevent infection or transmission.
Third, there is legitimate concern that the sub-optimal COVID vaccines might, in some people, actually facilitate entry of virus into human cells (when the vaccinated person is eventually exposed to the virus) and, thereby, enhance viral replication, disease severity, and spreading of the viral infection within the infected vaccinee and his/her contacts. [1074] This would result in the vaccinated individual becoming more severely ill than an unvaccinated person who has been exposed to the same amount of virus. [1074] There is legitimate concern, therefore, that when vaccinated people eventually become infected with SARS-CoV-2 they are more likely to become “spreaders” than are unvaccinated people who become infected. (More on this later.)
Nevertheless, when the COVID vaccines were first given temporary Emergency Use Authorization (EUA), they were celebrated as being spectacularly effective—exceeding expectations and representing a remarkable achievement of medical science. They were said to be in the range of “95% effective.” [1054] The public was given the impression that if you got vaccinated and subsequently got exposed to the virus, you had a 95% chance of either not getting infected or experiencing only mild infection. The vaccines were said to at least protect against severe infection. [1054] The further impression was that “of course”, a small percentage of vaccinated people (5%) would be expected to get COVID, “because the vaccine is only 95% effective, not 100% effective.”
But on what basis were these claims made. Let’s look at the clinical trial conducted by Pfizer: [1054]
In the Pfizer trial 21,720 people received the vaccine and 21,728 people received placebo. [1054] The trial primarily consisted of documenting how many in each group subsequently developed a positive COVID PCR test during the trial period, which lasted only 2-3 months. Elderly people, pregnant women, and children were excluded from the study.
Of the 43,448 people in the study, 170 became COVID PCR positive (0.4%)—162 in the placebo group (0.74%) and 8 in the vaccinated group (0.036%). However, the Ct values at which the positive PCR tests were positive were not reported, nor was confirmatory genomic sequencing performed. (See Section 6 for discussion of Ct values and genomic sequencing.) This means that we do not know how many of the COVID PCR positive people in each group had definite evidence of COVID. For example, in the placebo group, it is conceivable that 90% of the 162 PCR positive people (146 of the 162 people) had positive PCR results at Ct values greater than 30 and that genomic sequencing of all 146 people would have been negative—which would mean that none of those 146 people truly had COVID. And, in the vaccinated group, it is conceivable that 7 of the 8 PCR positive people were positive at a Ct value less than 30 and all of them would have been COVID positive upon genomic sequencing—which would mean that 7 of the 8 truly had COVID. Unfortunately, we do not know the Ct values at which the positive PCR tests were positive, and genomic sequencing was not done (or at least not reported). If these details were not taken into account, the results of the Pfizer study are scientifically unsound.
We also need to know how many people in each group had well-documented evidence of no prior infection with COVID—meaning that thorough and sophisticated tests for both B and T cell immunity to COVID were negative in all participants in the study. If a higher percentage of people in the vaccinated group had already acquired immunity from past COVID infection, then they would be less likely to develop COVID after vaccination, not necessarily because of the vaccination, but because of their past acquired immunity, at least in part. Adequately thorough testing for past infection was not done.
We also need to know with certainty whether factors such as crowded working conditions and other high exposure risks were equal in the two groups. For example, if participants in the vaccinated group were mostly suburban folks working from home, while participants in the placebo group were mostly working-class people working in crowded conditions, this could make a big difference in outcomes. Without these and many other details, the results of this Pfizer study should be interpreted with great caution.
The Pfizer study concluded that the vaccine provides a Relative Risk Reduction (RRR) of 95%. This is based on the following correctly used formula: Placebo Outcome minus Vaccine Outcome divided by Placebo Outcome times 100 equals the % RRR. That is: 162 minus 8 divided by 162 x 100 = 95% RRR. However, are we certain that this RRR of 95% necessarily means that only 5% of vaccinated people will be become infected when exposed to an infection-causing viral load?
Furthermore, when one analyses the Pfizer data in absolute terms, only 0.74% (a very small percentage) of the placebo group developed a positive PCR test, while 0.036% of the vaccinated group developed a positive PCR test. That is, less than 1% in either group developed a positive PCR test. (Again, without knowing the Ct values at which the PCR tests were positive, and without knowing the results of genomic sequencing, it is impossible to interpret these PCR results.) The vaccine reduced the incidence of PCR positivity from 0.74% to 0.036%. This represents an “Absolute Risk Reduction (ARR)” of 0.704% (0.74 minus 0.036) i.e., an ARR of less than 1%, which is not very impressive. Compared to the RRR, the ARR provides more realistic and appropriate guidance to an individual, regarding the extent to which a vaccine provides a meaningful risk reduction for that individual.
Since no subjects in the Pfizer study died, the study could not state that the Pfizer vaccine prevents deaths. Although there were said to be more instances of “severe illness” in the placebo group, more details and further/longer study are needed to determine whether these vaccines truly reduce the level of COVID disease severity and death.
The data produced by the Pfizer vaccine trial are not of sufficient scientific quality to permit solid conclusions regarding how truly effective these vaccines are in preventing or minimizing illness. Pfizer, the CDC, and Dr. Fauci have correctly admitted that these vaccines do not prevent virus from entering cells of vaccinated people, replicating there, and spreading to others—i.e., the vaccines do not prevent infection or transmission.
When one looks at the “real-world” experience with the COVID vaccines, one finds conflicting information, regarding the actual effectiveness of the vaccines. This is not surprising, considering the low scientific quality of the data being collected and reported (by the CDC, for example), as discussed in Section 6. Scientifically sound conclusions cannot be drawn, if the data are collected in a scientifically unsound fashion. That is a fundamental principle of medicine and scientific research, and, yet, that principle has been fundamentally violated in the collection, reporting, and interpretation of data, particularly at the level of state health departments and the CDC. (See Section 6.) Accordingly, such data must be interpreted with great caution. To be fair, all data, from all institutions and all countries must be critically examined and interpreted with caution, because of the problems pointed out in Section 6.
While keeping the above caveat in mind, we should be aware that many studies suggest that the COVID vaccines may be less effective than initially thought. [1055-1078] Instructive results have been reported in Israel, which was the first country to roll out a rapid and massive vaccination campaign (using the Pfizer vaccine) and among the first to achieve a high percentage of vaccination of its citizenry (approximately 80% as of September 2021). During the summer and fall of 2021, the predominant variant in Israel was the Delta variant. The following has been observed in Israel:
According to an independent analysis of official Israeli data [1058], vaccination appears to “fragilize” the immune system of the vaccinated person during the 5 weeks after the first vaccine dose is given. During this 5-week vaccination period, there appeared to be a 3-fold increase in COVID cases and a 20 x greater COVID death rate among the vaccinated, compared to the unvaccinated. [1058]
An Israeli study by Gazit et al revealed that vaccinated people who had had no prior evidence of natural SARS-CoV-2 infection were 13.04 times more likely to develop “breakthrough” infection with the Delta variant, compared to people who had previously been infected and were unvaccinated—suggesting that protection provided by naturally acquired immunity is superior to that provided by vaccine-induced immunity. [20]
Data from the UK have also suggested that an increased COVID death rate may be associated with COVID vaccination. Data published in a Public Health England briefing on September 3, 2021, revealed that, with the Delta variant, the death rate among unvaccinated individuals was 0.24%, while the death rate among all vaccinated (partial or full) individuals was 0.54%, and the death rate among fully vaccinated individuals was 0.96%. [1059] By August 2021, the Delta variant was accounting for 99% of sequenced cases of SARS-CoV-2 in England. Of the COVID deaths due to the Delta variant, 69% had received at least one vaccination dose, 61% had been fully vaccinated, and 30% were unvaccinated. [1059]
Data from the USA have been conflicting and difficult to interpret, primarily because of the extremely low scientific quality of much US data. (See Section 6.) A report by the CDC revealed that during the month of July 2021, an outbreak of 469 cases of COVID occurred in Barnstable County, Massachusetts. 74% of these 469 cases occurred in fully vaccinated individuals. [1060]
In a study in Viet Nam, many fully vaccinated hospital workers became infected with the delta variant. [1061] Nasal swabs from these workers revealed a viral load that was 251 times greater than the viral loads that had typically been seen in COVID cases during the pre-vaccine era. This suggests that the vaccination not only failed to prevent infection of these vaccinated health care workers, but might have enhanced viral replication within the vaccinated, converting them into super-spreaders. Those vaccinated workers were, indeed, shown to infect patients and unvaccinated co-workers.
Several other studies have documented failure of the vaccines to prevent the vaccinated from becoming infected, ill, and infectious (capable of transmitting infection to others). [1062, 1063] Hetemaki et al documented both asymptomatic and symptomatic infection among vaccinated health care workers and revealed that symptomatically infected health care workers, despite being vaccinated and using personal protective equipment, could transmit infection to others. [1062] Salvatore et al documented that of individuals with COVID in a federal penitentiary 82% had been fully vaccinated and only 18% were unvaccinated. [1063]
Many studies have shown that COVID vaccination does not necessarily decrease viral load or transmissibility. Acharya et al showed no significant difference in Ct values (an indirect indicator of viral load) in vaccinated people who had become infected versus unvaccinated people who had become infected. [1064] Riemersma et al also showed no difference in viral load when vaccinated people who had become infected were compared to unvaccinated people who had become infected. [1065, 1066] Furthermore, Riemersma et al documented elevated viral loads in 67% of vaccinated people who had become asymptomatically infected; while elevated viral loads were found in only 27% of unvaccinated people who had become asymptomatically infected. The Riemersama studies document that vaccinated people can become asymptomatically infected, harbor the virus, and unknowingly transmit the virus to others. Singanyanagam et al also documented that fully vaccinated individuals can become infected and have peak viral loads similar to the peak viral loads of unvaccinated individuals who have become infected. Singanyanagam further demonstrated that vaccinated individuals who become infected can efficiently transmit virus to fully vaccinated contacts. [151] Chia et al documented that Ct values at the time of diagnosis were similar in vaccinated and unvaccinated people who had become infected. [1067] These studies suggest that discrimination against unvaccinated people (giving privileges to the vaccinated and taking privileges away from the unvaccinated) is not only cruel, but scientifically unsound.
Many studies have shown that vaccinal antibody levels and vaccine efficacy decline steadily and substantially during the several months following vaccination: Chemaittelly et al showed that vaccine efficacy against infection waned to around 30% within 4-5 months after the second dose. [1068] Nordstrom et al showed that vaccine efficacy against infection decreased to 47% by the 4–6-month mark and was at 23% by the 7-month mark. [1069] Canaday showed that vaccine efficacy dropped more than 84% within 6 months after the second vaccine dose. Israel et al showed that vaccine-induced antibody titers declined by 40% each month, compared to a decline of less than 5% per month in unvaccinated people who had recovered from COVID. [1070] Eyre et al also documented that antibody levels in vaccinated individuals declined faster than did antibody levels in unvaccinated people who had experienced natural infection. [1071]
Disturbing results were reported by Yahi, et al, regarding the Delta variant and vaccination. They noted that vaccine-induced neutralizing antibodies had weak affinity for the spike protein while facilitating antibodies had stronger affinity. [1072] Facilitating antibodies facilitate entry of virus into cells. This means that in vaccinated individuals, the balance favors entry of virus into cells. This would be an example of how vaccination might predispose the vaccinated individual to ADE—antibody dependent enhancement (worsening) of infection, disease, and contagiousness.
The Omicron Variant: Several studies suggest that the COVID vaccines have failed even more since the Omicron variant has become predominant. Holm-Hansen et al have documented that, during the 4-5 months after vaccination, the Pfizer and Moderna vaccines have negative vaccine efficacy—meaning that vaccinated individuals (compared to the unvaccinated) are at increased risk of becoming infected with Omicron at the 4-5 month mark: [89] Four-five months after vaccination with Pfizer, the relative risk reduction (RRR, or “vaccine efficacy”) was minus 76.5%; after Moderna the RRR was minus 39.3%. [89] This finding supports the concern that these COVID vaccines can actually increase a person’s susceptibility to infection with Omicron.
Data provided by Public Health Scotland [1073] shows that during the last two weeks of December 2021 and the first 2 weeks of January 2022 people who had never been vaccinated were doing better than those who had been double-vaccinated: The unvaccinated had lower rates of COVID infection, COVID hospitalization, and COVID death than did the double-vaccinated. However, in that same report people who had received a booster dose appeared to have a lower rate of COVID death.
According to data from Ontario Health Services [1074], as of January 22, 2022, the majority of patients with COVID in Ontario hospitals and ICUs were fully vaccinated. In Australia, as of January 13, 2022, the majority of hospitalized COVID patients and ICU COVID patients were fully vaccinated. [1075]
Fortunately, the Omicron variant appears to cause milder illness for children than preceding variants. [1076]
The above studies suggest that the COVID vaccines, whose efficacy was not convincing in the first place, are now increasingly failing. They appear to actually increase risk of COVID infection and COVID death during the 5 weeks after the first dose; then there appears to be temporary and modest protection (at best) for a matter of only weeks or a few months; then there appears to be a negative effect (increased susceptibility to COVID infection). It is likely that Boosters will prove to provide only transient benefit, which is likely due to brief non-specific stimulation of natural immunity. Furthermore, the vaccines appear to be contributing to the development and spread of more worrisome variants; they may be predisposing vaccinated people to dangerous facilitating hyperimmune reactions (ADE) when they eventually become infected; and there is some evidence that vaccinated people may be more likely to spread the virus than are the unvaccinated.
So, both the short-term effectiveness and the long-term effectiveness of the COVID vaccines appear to be disappointing, especially with the newer variants. Natural immunity appears to be more robust and more durable than vaccine-induced immunity. [20, 40, 94, 141, 148]
On the other hand, the CDC and conventional media have continually reported that unvaccinated persons are at much higher risk for COVID infection, COVID hospitalization, and COVID death, when compared to vaccinated individuals. For example, a January, 21, 2022 MMWR report from the CDC by Johnson, et al states that during the months of October and December, 2021 unvaccinated persons had a 13.9 times higher risk of becoming infected with COVID and a 53.2 times higher risk of COVID death when compared to people who had been double-vaccinated and had received a booster dose; and unvaccinated persons had a 4 times greater risk of COVID infection and a 12.7 times greater risk of COVID death when compared to people who had been double vaccinated but had not received a booster: https://www.cdc.gov/mmwr/volumes/71/wr/mm7104e2.htm?s_cid=mm7104e2_w#suggestedcitation
For the reasons explained in Section 6, all data, from all institutions and all countries must be critically examined and interpreted with caution, including data from Johns Hopkins, WHO, the CDC and state health departments—because of the problems pointed out in Section 6. Frankly, because of the problematic way in which COVID data have been collected to date, I do not think we know how truly protective the COVID vaccines have been. Determination of true vaccine efficacy will require much more scientifically sound data collection and much more scientifically sound analysis than has occurred to date. [882]
The conflicting reports, contradictory data, and opposing views on COVID vaccination have made it very difficult for parents and physicians to make decisions about COVID vaccination. To analyze the enormous amount of complex, conflicting and confusing information, and to try to reach consensus, it would be wise to assemble an inclusive and diverse panel of fairly selected, internationally respected virologists, vaccinologists, epidemiologists, statisticians, and others to carefully, critically, respectfully, and publicly examine this issue. As part of informed consent, the public certainly deserves to know and needs to know the extent to which the COVID vaccines are effective, safe, and benefitting Humanity.
SECTION 8: HOW NECESSARY AND WISE HAS THE MASS COVID VACCINATION CAMPAIGN BEEN?
In Section 3 of this Open Letter, we explained how the COVID pandemic would likely have played out, if it had never been treated with vaccines: There would have been a certain number of deaths and serious COVID illnesses—far fewer, however, than occurred during 2020 (a year of clinical undertreatment) and 2021 (a year of mass vaccination plus undertreatment). Again, many of the deaths during 2020 and 2021 could have been prevented with more prompt and appropriately aggressive treatment and with better pandemic management overall:
See: https://notesfromthesocialclinic.org/treatment-of-severe-covid-19-illness-long-version/
Most of the serious illnesses and deaths (during a pandemic that was not treated with mass vaccination) would have occurred in the elderly and otherwise vulnerable. Children would have been least affected—only minimally affected. Many mutations and resultant variants would have developed during the course of the pandemic, but none would have become overwhelmingly predominant, and the variants would not have become as extremely transmissible as has occurred during the mass vaccination campaign. [1078] Considerable infection-acquired herd immunity would have developed, and it would be robust, sterilizing, and durable. The initial pandemic would have resolved within several months. Then, during the next winter, when COVID returns, it would be handled well, because of the robust natural herd immunity acquired during the first experience, and because of additional adjustments the immune system makes. In fact, what happens is that people with strong innate immunity continue to clear the virus with little or no symptoms whereas those who had to invoke some help from the acquired immune system will recall their previous antibodies, which, in most cases, are still quite cross-protective towards the next “natural” variant (unless an antigenic shift instead of drift occurred)
Unfortunately, the initial pandemic was not managed in the above fashion and has not played out as described above. Instead, particularly during 2021 (i.e., since onset of the mass vaccination campaign in December 2020), we have seen a prolongation of the pandemic, with an unusual number of waves. Several different predominating variants have sequentially developed. The vaccines have been failing. As the variants have become increasingly transmissible, both vaccinated and unvaccinated people are becoming infected. The vaccinated are carrying viral loads that are equal to or greater than the loads carried by the unvaccinated. Children are now becoming more affected than was the case during the initial wave—because of the increased transmissibility of the new variants and possibly because of vaccine-induced impairment of their natural innate immunity. The vaccinated are becoming increasingly vulnerable to ADE phenomena that threaten to enhance viral load, disease severity, and disease spread.
According to proponents of the alternative narrative, the mass vaccination campaign has prolonged the pandemic, impaired development of naturally acquired herd immunity, and made the pandemic worse for all concerned. Their contention is that we would be much better off now, if the mass vaccination campaign had never been implemented—including having fewer COVID hospitalizations and COVID deaths. Add to this the published reports and VAERS reports of large numbers of adverse reactions and deaths associated with COVID vaccination—reactions and deaths that would not have occurred in the absence of the vaccination campaign.
Add to this the fact that children were not at any high risk during the first year of the pandemic, during which time there was no vaccination available. As discussed (and referenced) in Section 5, it is rare for a previously healthy child to die from COVID itself. During the first 17 months of the COVID pandemic in Japan, no Japanese child died from COVID. During the first 15 months of the pandemic in Germany, no healthy German children between the ages of 5-12 died of COVID. During the first 4 months of the pandemic in Sweden, none of Sweden’s 1,951,905 children died of COVID. In England, during the first 12 months of the pandemic, 25 children and young people (CYP) died of COVID, out of a total population of 12,023,568 CYP, which translates to 1 COVID death per 480,000 CYP. In that English study 99.995% of CYP with a positive COVID test survived.
Add to this the fact that many vaccinated children have developed worrisome myocarditis and there have been COVID vaccine-related deaths in children. Evidence is mounting that, in children and adolescents, the frequency of vaccine-related myocarditis and vaccine-related death is greater than myocarditis and death due to properly treated natural COVID infection.
The above analysis suggests that, on balance, the mass COVID vaccination campaign (with its suboptimal, non-sterilizing vaccine) has caused more harm than good. It appears that we would have been better off at this point, if the mass vaccination campaign had never been implemented. It also appears that the best thing to do at this point is to stop the mass vaccination campaign and go back to managing the pandemic in the way that it should have been managed in the first place.
It also appears that if we do not stop the current mass vaccination campaign, we will, regrettably, experience more of the same failure—the pandemic will become even more prolonged and more dangerous; people will increasingly receive boosters, which might provide brief modest benefit (at best), but will increasingly fail and cause increasingly regrettable and cumulative side effects; the vaccinated will increasingly experience ADE, and children will increasingly become adversely affected by new vaccine-generated variants, by vaccine-mediated harm to their immune systems, by vaccine side effects, and by psycho-social-educational-economic disruptions.
In short, the risks associated with the mass vaccination campaign appear to outweigh any benefits. In short, our best current option is to stop the mass vaccination campaign immediately—at the very least for children—and manage the pandemic in the way it should have been managed in the first place. It is not too late to manage this pandemic properly.
However, as with other issues discussed in this Letter, it would be best to assemble an inclusive Independent International COVID Commission and ask panels of fairly selected, internationally respected virologists, vaccinologists, and epidemiologists to carefully, critically, respectfully, and publicly examine these issues. As part of informed consent, the public certainly deserves to know and needs to know the extent to which the mass COVD vaccination campaign has been necessary and wise.
SECTION 9: THE OBLIGATION OF PEDIATRICIANS TO PROVIDE SUFFICIENT INFORMATION FOR TRUE INFORMED CONSENT:
Having been a busy pediatrician and pediatric rheumatologist, in both academic medicine and private practice, I know how difficult it is for pediatricians to find the time and energy to research a new complex problem like the COVID pandemic. Most pediatricians, like most citizens, probably have not had time to thoroughly study the COVID situation for themselves. Instead, they have probably largely relied on and followed the recommendations of the prevailing narrative that has been strongly promoted by the US COVID Task Force, the CDC, and the conventional media.
Because I have been retired for the past 2 years, I have had the time and energy to thoroughly study the science behind the COVID situation, including the science of vaccination. Having a strong immunology background has helped me. This Open Letter shares what I have learned and temporarily concluded—until a proper COVID Commission is convened and scientists and physicians with greater expertise than mine engage in healthy dialogue. I hope this Open Letter will be helpful to those physicians who have had much less time to study the COVID situation.
If I were a practicing pediatrician who is being strongly urged by my institution, the CDC, NIH, WHO, Dr. Fauci, and the President of the United States to vaccinate as many children as possible, I would feel obligated to share the concerns mentioned in this Open Letter with the child’s parents. That is, I would feel obligated to provide opportunity for true informed consent, especially since these are incompletely studied experimental vaccines that are using a platform that has never before been used on human beings—especially because these vaccines are being advocated for children. I would want the child’s parents to be fully aware of all of the issues and concerns discussed in this Letter.
Many children have already been vaccinated and are now being strongly encouraged (even coerced, even forced) to receive booster doses. How many of those vaccinated children’s parents were adequately educated about the concerns discussed in this Open Letter? How much information did they receive before signing consent for their child’s vaccination? How many were simply, confidently, and only told, “These vaccines are very safe, very effective, and very necessary—just get vaccinated”?
Consent must be informed. Pediatricians are welcome to share this Open Letter with parents, as a resource for informed consent. Likewise, parents are welcome to share this Open Letter with family members, other parents, and the larger community.
SECTION 10: CONCLUSIONS:
- The prevailing COVID narrative has been primarily based on scientifically unsound use of the COVID PCR test and scientifically unsound collection of data, regarding COVID cases, COVID hospitalizations, COVID deaths, and vaccine efficacy.
- Scientifically unsound data collection leads to scientifically unsound conclusions and scientifically unsound public policies.
- The alternative COVID narrative represents a deep and sound scientific explanation that has been developed and articulated by extremely competent and caring scientists and physicians who appreciate the complexity and elegance of the immune ecosystem, have devoted their careers to the proper development and use of life-saving vaccines, and have dedicated the past 22 months to thoroughly studying the COVID situation. They are not “anti-vaxxers.” On the contrary, they are pro-vaccination but insist that vaccines be adequately demonstrated to be safe, effective, and necessary.
- According to the alternative narrative, and in my opinion as well, the COVID vaccines are inadequately safe, inadequately effective, and have been doing more harm than good.
- The mass vaccination campaign, with its sub-optimal (non-sterilizing) vaccines, has prolonged the pandemic, generated more transmissible and potentially more lethal variants, has predisposed the vaccinated to life-threatening ADE, has transformed the vaccinated into major spreaders of the virus, has disrupted the normal healthy flow of immune system function (i.e., has harmfully disturbed the normal immune ecosystem, particularly the immature innate immunity of children), and has greatly interfered with development of sterilizing herd immunity. The result has been more deaths and hospitalizations than would have occurred if the COVID pandemic had been treated without this mass vaccination campaign.
- This has not been a “pandemic of the unvaccinated,” it has been a pandemic that has been prolonged and made worse by an ill-conceived mass vaccination campaign.
- In addition to being enormously problematic at a population level, the COVID vaccines have caused life-threatening side effects at the individual level.
- Furthermore, the COVID vaccines have been far less effective than claimed by their manufacturers and the CDC. At best they might provide transient benefit, and even that benefit is questionable.
- The risks associated with COVID vaccination (at both the population level and the individual level) appear to substantially outweigh the benefits—particularly in children and even in the elderly and frail.
- Moreover, throughout the pandemic, people who have become infected have commonly been undertreated—receiving neither proper early outpatient treatment, nor proper inpatient treatment. This has added to the hospitalizations and deaths that could have been prevented.
- The COVID vaccines have been promoted without proper informed consent. Neither the public, nor parents, have been provided adequate information by the proponents of the prevailing narrative—regarding vaccine side effects suffered by individuals and adverse consequences of the mass vaccination campaign at the population level. Instead, they have been given simplistic, falsely reassuring, one-sided information and have been told that the information provided by the alternative narrative (the science-based, data-driven alternative narrative described in this Letter) represents “misinformation.”
- Parents and the public deserve to have representatives of the two opposing COVID narratives come together to engage in healthy, respectful, scientifically sound, publicly witnessed (televised) dialogue about the safety, efficacy, necessity, results, and wisdom of the current COVID mass vaccination campaign. For 22 months, leaders of the alternative narrative have been pleading for such, to no avail.
- Parents and the public can and should insist that an “Inclusive Independent International COVID Commission” be formed, consisting of independent international panels of fairly selected, eminent scientists who would be asked to thoroughly and objectively address COVID vaccination issues in an effort to resolve disagreements and arrive at consensus. Such is the tradition of science, medicine, democracy, and civil society. The public deserves and desperately needs such careful examination and healthy dialogue. Successful resolution of the COVID pandemic depends on it.
- Until the above-suggested Commission convenes and arrives at a thoughtful and fair consensus, the current mass vaccination campaign should—out of an abundance of caution—be at least temporarily suspended, at least for children.
- As parents and the public watch C-SPAN-like televised proceedings of the Commission, they (parents and the public) can decide in their own minds who among the Commissioners and discussants seems most knowledgeable, careful, rigorously scientific, compassionate, honest, ethical, and most wise.
- Only then will parents be able to make a truly informed decision for their children.
- As a pediatrician, a father, and a grandfather, I must do my part to protect the integrity of the developing immune ecosystem in children and to protect Humanity from the adverse effects of an ill-conceived mass vaccination campaign. Hence, this Open Letter.
- For the sake of our children, grandchildren, and all of humanity, we have an individual and collective social responsibility to call for an immediate and complete halt to the current COVID vaccination campaign, on a scientific basis alone, until an appropriate COVID Commission is convened to thoroughly and accurately evaluate the COVID situation. In the meantime, current scientific evidence strongly suggests that to participate in the continuation of the COVID vaccination campaign—-to promote it, to remain silent about it, or to personally receive further COVID vaccination—-is to contribute to the harm of children and humanity, as well as harm to oneself.
- Morally, ethically, and scientifically, we have a social responsibility to call for at least temporary cessation of the COVID vaccination campaign. Such a call is an unselfish, science-based act of courage and social responsibility, behind which all of humanity (whether currently unvaccinated or already vaccinated) can confidently unite, to the mutual support and the emotional, social, and health benefit of all.
ACKNOWLEDGMENTS: I would like to thank all of the scientists and physicians who have cared and dared to share their deep concerns about the prevailing COVID narrative. In particular, I would like to thank Dr. Geert Vanden Bossche for sharing his extraordinary understanding of immunology, virology, vaccinology, and evolutionary biology with all of us, and for providing me with his invaluable and careful review of this Open Letter.

What is this country doctor thinking? Is he wondering how much longer he can continue to attend to all the patients who need him? Is he wondering whether the medications he needs will continue to be available and permitted use? Will there be enough nurses to help him? Are the days of practicing Medicine in the meaningful and rewarding way he had always practiced now over? What does the future hold for Medicine and Humanity?

This is Albert Schweitzer (1875-1965), hard at work trying to figure out how to best meet his patients’ needs. He was a theologian and philosopher, as well as a physician. In 1952 he was awarded a Nobel Prize for his philosophy of “Reverence for Life.” He devoted his life to caring for patients in Africa, at the Albert Schweitzer Hospital in Lambarene, Gabon. He was one of the first Europeans to believe and act on the notion that African lives mattered. What would Dr. Schweitzer think of the COVID pandemic and how it has been handled—scientifically, ethically, and morally?
LINKS TO EDUCATIONAL VIDEO INTERVIEWS AND PRESENTATIONS:
- Geert Vanden Bossche: Mass Vaccination in a Pandemic: https://odysee.com/@bonniesmit:0/Mass-Vaccination-in-a-Pandemic—Geert-Vanden-Bossche:a
- Geert Vanden Bossche: Part 1. Children Vaccination: https://www.voiceforscienceandsolidarity.org/videos-and-interviews/part-1-children-vaccination-english
- Geert Vanden Bossche: Part 2-3. Booster and Omicron Booster: https://www.voiceforscienceandsolidarity.org/videos-and-interviews/part-2-3-booster-and-omicron-booster
- Geert Vanden Bossche: Part 4. Who is Geert? https://www.voiceforscienceandsolidarity.org/videos-and-interviews/part-4-who-is-geert
- Geert Vanden Bossche: Second Call to the WHO: Please don’t vaccinate against Omicron. https://www.voiceforscienceandsolidarity.org/videos-and-interviews/second-call-to-who-please-dont-vaccinate-against-omicron
- Geert Vanden Bossche: Geert’s New Year Message: What’s next in 2022? https://www.voiceforscienceandsolidarity.org/videos-and-interviews/geerts-new-year-message-whats-next-in-2022
- Geert Vanden Bossche: On Natural Immunity being Safer for Children: https://www.voiceforscienceandsolidarity.org/videos-and-interviews/unforgivable-sin-dr-geert-vanden-bossche-on-natural-immunity-being-safer-for-children
- Geert Vanden Bossche: The Implication of Mass Vaccination During the Pandemic: https://www.voiceforscienceandsolidarity.org/videos-and-interviews/the-implication-of-massive-vaccination-during-the-pandemic
- Geert Vanden Bossche: COVID-19 Vaccine Mandates for Children; What is the Science behind this? https://www.voiceforscienceandsolidarity.org/videos-and-interviews/covid-19-vaccine-mandates-for-children
- Sucharit Bhakdi: An Open Letter to the Chancellor of Germany: https://video.search.yahoo.com/search/video;_ylt=Awr9DuoqcvBhR34Ad3tXNyoA;_ylu=Y29sbwNncTEEcG9zAzEEdnRpZAMEc2VjA3Nj?p=youtube+prof+sucharit+bhakdi&fr=mcafee#id=52&vid=ba401c9825681e020f2cf6af4abddf6f&action=view
- Sucharit Bhakdi: Do we need a vaccine against COVID-19? https://video.search.yahoo.com/search/video;_ylt=Awr9DuoqcvBhR34Ad3tXNyoA;_ylu=Y29sbwNncTEEcG9zAzEEdnRpZAMEc2VjA3Nj?p=youtube+prof+sucharit+bhakdi&fr=mcafee#id=8&vid=40e0143c82fe2259786f16a194f1889a&action=view
- Sucharit Bhakdi: Massive self-to-self attack of immune system. Note: Start at the 2:46 minute mark: https://rairfoundation.com/dr-sucharit-bhakdi-vaccine-benefit-zero-fears-massive-self-to-self-attack-of-immune-system-video/
- Sucharit Bhakdi: Doctors for COVID Ethics—Symposium II, December 10, 2021. View Dr. Bhakdi’s closing emotional plea to the world’s physicians to protect children and humanity from the dangers of the COVID vaccines (starting at the 3:47:07 mark): https://doctors4covidethics.org/gold-standard-covid-science-in-practice-interdisciplinary-symposium-ii-december-10-2021/
- Dr. Arne Burkhardt: 2nd Pathology Conference—Are Deaths and Adverse Health Effects after Vaccination against COVID-19 related in a Pathologically detectable way? https://odysee.com/@en:a5/Pathology-Conference-2-en:b Note: The most important portion of Dr. Burkhardt’s presentation extends from the 16:30 minute mark to the 52:36-minute mark.
- Geert Vanden Bossche: Predictions on Evolution of COVID 19 Pandemic. https://www.voiceforscienceandsolidarity.org/scientific-blog/predictions-gvb-on-evolution-c-19-pandemic
- Russell MW, Moldoveanu Z, Ogra PL and Mestecky J (2020) Mucosal Immunity in COVID-19: A Neglected but Critical Aspect of SARS-CoV-2 Infection. Front. Immunol. 11:611337. doi: 10.3389/fimmu.2020.611337
- Russell MW, Mestecky J, Strober W, Kelsall BL, Lambrecht BN, Cheroutre H. The mucosal immune system: Overview. In: J Mestecky, W Strober, MW Russell, BL Kelsall, H Cheroutre, BN Lambrecht, editors. Mucosal Immunology, 4. Amsterdam: Academic Press/Elsevier (2015). p. 3–8.
- Russell MW. Biological functions of IgA. In: CS Kaetzel, editor. Mucosal Immune Defense: Immunoglobulin A. New York: Springer (2007). p. 144–72.
- Baker K, Blumberg RS, Kaetzel CS. Immunoglobulin transport and immunoglobulin receptors. In: J Mestecky, W Strober, MW Russell, BL Kelsall, H Cheroutre, BN Lambrecht, editors. Mucosal Immunology, 4. Amsterdam: Academic Press/Elsevier (2015). p. 349–407.
- Kubagawa H, Bertoli LF, Barton JC, Koopman WJ, Mestecky J, Cooper MD. Analysis of paraprotein transport into saliva by using anti-idiotype antibodies. J Immunol (1987) 138:435–9.
- Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, et al. Immunology of COVID-19: current state of the science. Immunity (2020) 52:910–41. doi: 10.1016/j.immuni.2020.05.002
- Conley ME, Delacroix DL. Intravascular and mucosal immunoglobulin A: Two separate but related systems of immune defense? Ann Int Med (1987) 106:892–9. doi: 10.7326/0003-4819-106-6-892
- Russell MW, Kilian M, Mantis NJ, Corthé sy B. Biological activities of mucosal immunoglobulins. In: J Mestecky, W Strober, MW Russell, BL Kelsall, H Cheroutre, BN Lambrecht, editors. Mucosal Immunology, 4. Amsterdam: Academic Press/Elsevier (2015). p. 429–54.
- Bidgood SR, Tam JC, McEwan WA, Mallery DL, James LC. Translocalized IgA mediates neutralization and stimulates innate immunity inside infected cells. Proc Natl Acad Sci USA (2014) 111(37):13463–8. doi: 10.1073/ pnas.1410980111.
- Jackson S, Mestecky J, Moldoveanu Z, Spearman P. Appendix I: Collection and processing of human mucosal secretions. In: J Mestecky, J Bienenstock, ME Lamm, L Mayer, JR McGhee, W Strober, editors. Mucosal Immunology, 3. Amsterdam: Elsevier/Academic Press (2005). p. 1829–39
- Reyneveld GI, Savelkoul HFJ and Parmentier HK (2020) Current Understanding of Natural Antibodies and Exploring the Possibilities of Modulation Using Veterinary Models. A Review. Front. Immunol. 11:2139. doi: 10.3389/fimmu.2020.02139
- Coutinho A, Kazatchkine MD, Avrameas S. Natural autoantibodies. Curr Opin Immunol. (1995) 7:812–8. doi: 10.1016/0952-7915(95)80053-0
- Holodick NE, Rodríguez-Zhurbenko N, Hernández AM. Defining natural antibodies. Front Immunol. (2017) 8:872. doi: 10.3389/fimmu.2017.00872
- Panda S, Ding JL. Natural antibodies bridge innate and adaptive immunity. J Immunol. (2015) 194:13–20. doi: 10.4049/jimmunol.1400844
- Ochsenbein AF, Zinkernagel RM. Natural antibodies and complement link innate and acquired immunity. Immunol Today. (2000) 21:624–30. doi: 10.1016/s0167-5699(00)01754-0
- Binder C. Naturally occurring IgM antibodies to oxidation-specific epitopes. Adv Exp Med Biol. (2012) 750:2–13. doi: 10.1007/978-1-4614-3461-0_1
- Loske, J., Röhmel, J., Lukassen, S. et al. Pre-activated antiviral innate immunity in the upper airways controls early SARS-CoV-2 infection in children. Nat Biotechnol (2021). https://doi.org/10.1038/s41587-021-01037-9
- Shrestha, et al. Necessity of COVID-19 vaccination in previously infected individuals. 2021.
- Le Bert, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. 2020.
- Gazit, et al. Comparing SARS-CoV-2 natural immunity to vaccine-induced immunity: reinfections versus breakthrough infections. 2021.
- Le Bert, et al. Highly functional virus-specific cellular immune response in asymptomatic SARS-CoV-2 infection, 2021.
- Israel, et al. Large-scale study of antibody titer decay following BNT162b2 mRNA vaccine or SARS-CoV-2 infection, 2021.
- Pilz, et al. SARS-CoV-2 re-infection risk in Austria, 2021.
- Neidleman, et al. mRNA vaccine-induced SARS-CoV-2-specific T cells recognize B.1.1.7 and B.1.351 variants but differ in longevity and homing properties depending on prior infection status, 2021.
- Bhandari, et al. Good news: Mild COVID-19 induces lasting antibody protection, 2021.
- Wajnberg, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months, 2021
- Gaebler, et al. Evolution of Antibody Immunity to SARS-CoV-2, 2020
- Haveri, et al. Persistence of neutralizing antibodies a year after SARS-CoV-2 infection in humans, 2021
- Murchu, et al. Quantifying the risk of SARS‐CoV‐2 reinfection over time, 2021
- Nielsen, et al. SARS-CoV-2 elicits robust adaptive immune responses regardless of disease severity, 2021
- Goldberg, et al. Protection of previous SARS-CoV-2 infection is similar to that of BNT162b2 vaccine protection: A three-month nationwide experience from Israel, 2021
- Kojima, et al. Incidence of Severe Acute Respiratory Syndrome Coronavirus-2 infection among previously infected or vaccinated employees, 2021
- Wadman, et al. Having SARS-CoV-2 once confers much greater immunity than a vaccine—but vaccination remains vital, 2021
- Zhang, et al. One-year sustained cellular and humoral immunities of COVID-19 convalescents, 2021
- Rodda, et al. Functional SARS-CoV-2-Specific Immune Memory Persists after Mild COVID-19, 2021
- Ivanova, et al. Discrete Immune Response Signature to SARS-CoV-2 mRNA Vaccination Versus Infection, 2021
- Turner, et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans, 2021
- Hall VJ, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN), 2021
- Houlihan, et al. Pandemic peak SARS-CoV-2 infection and seroconversion rates in London frontline health-care workers, 2020
- Lumley, et al. Antibodies to SARS-CoV-2 are associated with protection against reinfection, 2021
- Cohen, et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells, 2021
- Sureshchandra, et al. Single cell profiling of T and B cell repertoires following SARS-CoV-2 mRNA vaccine, 2021
- Abu-Raddad, et al. SARS-CoV-2 antibody-positivity protects against reinfection for at least seven months with 95% efficacy, 2021
- Ripperger, et al. Orthogonal SARS-CoV-2 Serological Assays Enable Surveillance of Low-Prevalence Communities and Reveal Durable Humoral Immunity, 2020
- Wei, et al. Anti-spike antibody response to natural SARS-CoV-2 infection in the general population, 2021
- Yu. Researchers find long-lived immunity to 1918 pandemic virus, CIDRAP, 2008 and the actual 2008 NATURE journal publication by Yu
- Gonzalez, et al. Live virus neutralisation testing in convalescent patients and subjects vaccinated against 19A, 20B, 20I/501Y.V1 and 20H/501Y.V2 isolates of SARS-CoV-2, 2021
- Camara, et al. Differential effects of the second SARS-CoV-2 mRNA vaccine dose on T cell immunity in naïve and COVID-19 recovered individuals, 2021
- Klausner, et al. Op-Ed: Quit Ignoring Natural COVID Immunity, 2021
- Harvey, et al. Association of SARS-CoV-2 Seropositive Antibody Test With Risk of Future Infection, 2021
- Letizia, et al. SARS-CoV-2 seropositivity and subsequent infection risk in healthy young adults: a prospective cohort study, 2021
- Bertollini, et al. Associations of Vaccination and of Prior Infection With Positive PCR Test Results for SARS-CoV-2 in Airline Passengers Arriving in Qatar, 2021
- Mishra, et al. Natural immunity against COVID-19 significantly reduces the risk of reinfection: findings from a cohort of sero-survey participants, 2021
- NIH: Lasting immunity found after recovery from COVID-19, 2021
- Petersen, et al. SARS-CoV-2 Natural Antibody Response Persists for at Least 12 Months in a Nationwide Study From the Faroe Islands, 2021
- Jung, et al. SARS-CoV-2-specific T cell memory is sustained in COVID-19 convalescent patients for 10 months with successful development of stem cell-like memory T cells, 2021
- Ansari, et al. Immune Memory in Mild COVID-19 Patients and Unexposed Donors Reveals Persistent T Cell Responses After SARS-CoV-2 Infection, 2021
- WHO: COVID-19 natural immunity, 2021
- Cho, et al. Antibody Evolution after SARS-CoV-2 mRNA Vaccination, 2021
- Gudbjartsson, et al. Humoral Immune Response to SARS-CoV-2 in Iceland, 2020
- Dan, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection, 2021
- Chivese, et al. The prevalence of adaptive immunity to COVID-19 and reinfection after recovery – a comprehensive systematic review and meta-analysis of 12 011 447 individuals, 2021
- Sheehan, et al. Reinfection Rates among Patients who Previously Tested Positive for COVID-19: a Retrospective Cohort Study, 2021
- Vitale, et al. Assessment of SARS-CoV-2 Reinfection 1 Year After Primary Infection in a Population in Lombardy, Italy, 2020
- Hanrath, et al. Prior SARS-CoV-2 infection is associated with protection against symptomatic reinfection, 2021
- Grifoni, et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals, 2020
- Collins, et al. NIH Director’s Blog: Immune T Cells May Offer Lasting Protection Against COVID-19, 2021
- Wang, et al. Ultrapotent antibodies against diverse and highly transmissible SARS-CoV-2 variants, 2021
- Makary M. Why COVID-19 Vaccines Should Not Be Required for All Americans, 2021
- Ma, et al. Protracted yet coordinated differentiation of long-lived SARS-CoV-2-specific CD8+ T cells during COVID-19 convalescence, 2021
- Naniche, et al. Decrease in Measles Virus-Specific CD4 T Cell Memory in Vaccinated Subjects, 2004
- Palm, et al. Remembrance of Things Past: Long-Term B Cell Memory After Infection and Vaccination, 2019
- Lyski, et al. SARS-CoV-2 specific memory B-cells from individuals with diverse disease severities recognize SARS-CoV-2 variants of concern, 2021
- Wang, et al. Exposure to SARS-CoV-2 generates T-cell memory in the absence of a detectable viral infection, 2021
- Redd, et al. CD8+ T-Cell Responses in COVID-19 Convalescent Individuals Target Conserved Epitopes From Multiple Prominent SARS-CoV-2 Circulating Variants, 2021 and Lee, 2021
- La Jolla, et al. Exposure to common cold coronaviruses can teach the immune system to recognize SARS-CoV-2, 2020
- Mateus, et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans, 2020
- Dehgani-Mobaraki, et al. Longitudinal observation of antibody responses for 14 months after SARS-CoV-2 infection, 2021
- Juno, et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19, 2020
- Robbiani, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals, 2020
- Hartley, et al. Rapid generation of durable B cell memory to SARS-CoV-2 spike and nucleocapsid proteins in COVID-19 and convalescence, 2020
- Callaway, et al. Had COVID? You’ll probably make antibodies for a lifetime, 2021
- Majdoubi, et al. A majority of uninfected adults show preexisting antibody reactivity against SARS-CoV-2, 2021
- Braun, et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19, 2020
- Braun, et al. Presence of SARS-CoV-2-reactive T cells in COVID-19 patients and healthy donors, 2020
- Wang, et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection, 2021
- Rank, et al. One Year after Mild COVID-19: The Majority of Patients Maintain Specific Immunity, But One in Four Still Suffer from Long-Term Symptoms, 2021
- CDC: IDSA, 2021
- Holm Hansen, et al. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study, 2021
- Moderbacher, et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity, 2020
- Ni, et al. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals, 2020
- Zuo, et al. Robust SARS-CoV-2-specific T-cell immunity is maintained at 6 months following primary infection, 2020
- Tarke, et al. Negligible impact of SARS-CoV-2 variants on CD4+ and CD8+ T cell reactivity in COVID-19 exposed donors and vaccinees, 2021
- Perez, et al. A 1 to 1000 SARS-CoV-2 reinfection proportion in members of a large healthcare provider in Israel: a preliminary report, 2021
- Iyer, et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients, 2020
- Alfego, et al. A population-based analysis of the longevity of SARS-CoV-2 antibody seropositivity in the United States, 2021
- Hellerstein, et al. What are the roles of antibodies versus a durable, high- quality T-cell response in protective immunity against SARS-CoV-2? 2020
- Peng, et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent COVID-19 patients, 2020
- Sekine, et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19, 2020
- Lafone, et al. Potent SARS-CoV-2-Specific T Cell Immunity and Low Anaphylatoxin Levels Correlate With Mild Disease Progression in COVID-19 Patients, Lafron, 2021
- Nelde, et al. SARS-CoV-2 T-cell epitopes define heterologous and COVID-19 induced T-cell recognition, 2020
- Sayers, et al. Karl Friston: up to 80% not even susceptible to Covid-19, 2020
- Lineburg, et al. CD8+ T cells specific for an immunodominant SARS-CoV-2 nucleocapsid epitope cross-react with selective seasonal coronaviruses, 2021
- Saini, et al. SARS-CoV-2 genome-wide mapping of CD8 T cell recognition reveals strong immunodominance and substantial CD8 T cell activation in COVID-19 patients, 2020
- Shenai, et al. Equivalency of Protection from Natural Immunity in COVID-19 Recovered Versus Fully Vaccinated Persons: A Systematic Review and Pooled Analysis, 2021
- Satwik, et al. ChAdOx1nCoV-19 effectiveness during an unprecedented surge in SARS CoV-2 infections, 2021
- Molodtsoy, et al. SARS-CoV-2 specific T cells and antibodies in COVID-19 protection: a prospective study, 2021
- Cho, et al. Anti- SARS-CoV-2 Receptor Binding Domain Antibody Evolution after mRNA Vaccination, 2021
- Ortega, et al. Seven-month kinetics of SARS-CoV-2 antibodies and role of pre-existing antibodies to human coronaviruses, 2021
- Mahajan, et al. Immunodominant T-cell epitopes from the SARS-CoV-2 spike antigen reveal robust pre-existing T-cell immunity in unexposed individuals, 2021
- Wang, et al. Neutralizing Antibody Responses to Severe Acute Respiratory Syndrome Coronavirus 2 in Coronavirus Disease 2019 Inpatients and Convalescent Patients, 2020
- Cox, et al. Not just antibodies: B cells and T cells mediate immunity to COVID-19, 2020
- Di Piazza, et al. T cell immunity to SARS-CoV-2 following natural infection and vaccination, 2020
- Ogega, et al. Durable SARS-CoV-2 B cell immunity after mild or severe disease, 2021
- Ng, et al. Memory T cell responses targeting the SARS coronavirus persist up to 11 years post-infection., 2016
- Sette, et al. Adaptive immunity to SARS-CoV-2 and COVID-19, 2021
- Tan, et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients, 2021
- Kared, et al. SARS-CoV-2–specific CD8+ T cell responses in convalescent COVID-19 individuals, 2021
- Nguyen-Contant, et al. S Protein-Reactive IgG and Memory B Cell Production after Human SARS-CoV-2 Infection Includes Broad Reactivity to the S2 Subunit, 2021
- Yao, et al. Persistence of Antibody and Cellular Immune Responses in Coronavirus Disease 2019 Patients Over Nine Months After Infection, 2021
- De Giorgi, et al. Naturally Acquired SARS-CoV-2 Immunity Persists for Up to 11 Months Following Infection, 2021
- Smetana, et al. Decreasing Seroprevalence of Measles Antibodies after Vaccination – Possible Gap in Measles Protection in Adults in the Czech Republic, 2017
- Wrammert, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection, 2011
- Quereshi, et al. Reinfection With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Patients Undergoing Serial Laboratory Testing, 2021
- Goel, et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination, 2021.
- Doshi, et al. Covid-19: Do many people have pre-existing immunity? 2020
- Ng, et al. Pre-existing and de novo humoral immunity to SARS-CoV-2 in humans, Ng, 2020
- Weiskopf, et al. Phenotype of SARS-CoV-2-specific T-cells in COVID-19 patients with acute respiratory distress syndrome, 2020
- Sette, et al. Pre-existing immunity to SARS-CoV-2: the knowns and unknowns, 2020
- Greenbaum, et al. Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population, 2009
- Sridhar, et al. Cellular immune correlates of protection against symptomatic pandemic influenza, 2013
- Wilkinson, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans, 2012
- CDC, MMWR: Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine, 2009
- Welsh, et al. No one is naive: the significance of heterologous T-cell immunity, 2002
- Gallais, et al. Intrafamilial Exposure to SARS-CoV-2 Induces Cellular Immune Response without Seroconversion, Gallais, 2020
- Kojima, et al. Protective immunity after recovery from SARS-CoV-2 infection, 2021
- Kwon, et al. This ‘super antibody’ for COVID fights off multiple coronaviruses, 2021
- Wu, et al. SARS-CoV-2 infection induces sustained humoral immune responses in convalescent patients following symptomatic COVID-19, 2020
- Isho, et al. Evidence for sustained mucosal and systemic antibody responses to SARS-CoV-2 antigens in COVID-19 patients, 2020
- Bertoletti, et al. The T-cell response to SARS-CoV-2: kinetic and quantitative aspects and the case for their protective role, 2021
- Eyran, et al. The longitudinal kinetics of antibodies in COVID-19 recovered patients over 14 months, 2020
- Lan, et al. Continued Effectiveness of COVID-19 Vaccination among Urban Healthcare Workers during Delta Variant Predominance, 2021
- Sarraf, et al. Immunity to COVID-19 in India through vaccination and natural infection, 2021
- Garrido, et al. Asymptomatic or mild symptomatic SARS-CoV-2 infection elicits durable neutralizing antibody responses in children and adolescents, 2021
- Shrotri, et al. T cell response to SARS-CoV-2 infection in humans: A systematic review, 2021
- Abu-Raddad, et al. Severity of SARS-CoV-2 Reinfections as Compared with Primary Infections, 2021
- Abu-Raddad, et al. Assessment of the Risk of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Reinfection in an Intense Re-exposure Setting, 2021
- Andeweg, et al. Increased risk of infection with SARS-CoV-2 Beta, Gamma, and Delta variant compared to Alpha variant in vaccinated individuals, 2021
- Breathnach, et al. Prior COVID-19 protects against reinfection, even in the absence of detectable antibodies, 2021
- Rosenberg, et al. Natural infection vs vaccination: Which gives more protection?, 2021
- Singanayagam, et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study, 2021
- Greaney, et al. Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection, 2021
- Moderbacker, et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity, 2020
- Goldberg, et al. Protection and waning of natural and hybrid COVID-19 immunity, 2021
- Kojima, et al. A Systematic Review of the Protective Effect of Prior SARS-CoV-2 Infection on Repeat Infection, 2021
- Pape, et al. High-affinity memory B cells induced by SARS-CoV-2 infection produce more plasmablasts and atypical memory B cells than those primed by mRNA vaccines, 2021
- Chen, et al. Differential antibody dynamics to SARS-CoV-2 infection and vaccination, 2021.
- Dowell, et al. Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection, 2022
- Abu-Raddad, et al. Severity of SARS-CoV-2 Reinfections as Compared with Primary Infections, 2021
- Keeton, et al. SARS-CoV-2 spike T cell responses induced upon vaccination or infection remain robust against Omicron, 2021
- Greenbaum, et al. Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population, 2009
- Altarawneh, et al. Protection afforded by prior infection against SARS-CoV-2 reinfection with the Omicron, variant, 2021
- Kundu, et al. Cross-reactive memory T cells associate with protection against SARS-CoV-2 infection in COVID-19 contacts, 2022
- Vardhana, S.A., and J.D. Wolchok. 2020. The many faces of the anti-COVID immune response. J. Exp. Med. 217:e20200678. https://doi.org/10.1084/ jem.20200678
- Kuri-Cervantes, L., M.B. Pampena, W. Meng, A.M. Rosenfeld, C.A.G. Ittner, A.R. Weisman, R.S. Agyekum, D. Mathew, A.E. Baxter, L.A. Vella, et al. 2020. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci. Immunol. 5:eabd7114–20. https://doi.org/10 .1126/sciimmunol.abd7114
- Silvin, A., N. Chapuis, G. Dunsmore, A.-G. Goubet, A. Dubuisson, L. Derosa, C. Almire, C. Henon, O. Kosmider, N. Droin, et al. 2020. Elevated Cal-protectin and Abnormal Myeloid Cell Subsets Discriminate Severe from Mild COVID-19. Cell. 182:1401–1418.e18. https://doi.org/10.1016/j.cell .2020.08.002
- Nienhold, R., Y. Ciani, V.H. Koelzer, A. Tzankov, J.D. Haslbauer, T. Menter, N. Schwab, M. Henkel, A. Frank, V. Zsikla, et al. 2020. Two distinct immunopathological profiles in autopsy lungs of COVID-19. Nat. Commun. 11:5086. https://doi.org/10.1038/s41467-020-18854-2
- Laing, A.G., A. Lorenc, I. Del Molino Del Barrio, A. Das, M. Fish, L. Monin, M. Muñoz-Ruiz, D.R. McKenzie, T.S. Hayday, I. Francos-Quijorna, et al. 2020. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat. Med. 26:1623–1635. https://doi.org/10.1038/ s41591-020-1038-6
- Rydyznski Moderbacher, C., S.I. Ramirez, J.M. Dan, A. Grifoni, K.M. Hastie, D. Weiskopf, S. Belanger, R.K. Abbott, C. Kim, J. Choi, et al. 2020. Antigen Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell. 183:996–1012.e19. https://doi.org/10.1016/j.cell.2020.09.038
- Henderson LA, Canna SW, Schulert GS, et al. On the alert for cytokine storm: immunopathology in COVID-19. Arthritis Rheumatol 2020; published online April 15. DOI:10.1002/art.41285.
- Qin C, Zhou L, Hu Z, Zhang S, Yang S et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clinical Infectious Diseases: an official publication of the Infectious Diseases Society of America 2020. doi: 10.1093/ cid/ciaa248
- Wang W, He J, Lie p, Huang l, Wu S et al. The definition and risks of cytokine release syndrome-like in 11 COVID-19-infected pneumonia critically ill patients: disease characteristics and retrospective analysis. MedRxiv 2020. doi: 10.1101/2020.02.26.20026989
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395: 1033–34.
- Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Seminars in Immunopathology 2017; 39 (5): 529-539. doi: 10.1007/s00281-017-0629-x
- Shareef KA, et al. Cytokine Blood Filtration Responses in COVID-19. Blood Purification. Published online: May 28, 2020.
- Cron RQ, Chatham WW. The rheumatologist’s role in COVID-19. J Rheumatol 2020; 47: 639–42.
- Halpert G, Shoenfeld Y. SARS-CoV-2, the autoimmune virus. Autoimmunity Reviews 19 (2020) 102695. https://doi.org/10.1016/j.autrev.2020.102695
- Shoenfeld Y. Corona (COVID-19) time musings: Our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmunity Reviews 19 (2020) 102538. https://doi.org/10.1016/j.autrev.2020.102538
- Ruscitti P, Berardicurti O, Di Benedetto P, et, al. (2020) Severe COVID-19, Another Piece in the Puzzle of the Hyperferritinemic Syndrome. An Immunomodulatory Perspective to Alleviate the Storm. Front. Immunol. 11:1130. doi: 10.3389/fimmu.2020.01130
- Ryabkova VA, Churilov LP, Shoenfeld Y. Influenza infection, SARS, MERS and COVID-19: Cytokine storm – The common denominator and the lessons to be learned. Clinical Immunology 223 (2021) 108652 https://doi.org/10.1016/j.clim.2020.108652
- Rennebohm RM. Has undertreatment of severe COVID illness been widespread? A pediatric rheumatologist’s perspective. Russia Biomedical Research, 2020, Vol 5, No 3, p. 3-13.
- McCullough PA, Kelly RJ, Ruocco G, et al. Pathophysiological Basis and Rationale for Early Outpatient Treatment of SARS-CoV-2 (COVID-19) Infection. Am J Med. 2021;134(1):16-22. doi:10.1016/j.amjmed.2020.07.003
- H.A. Risch. “Early Outpatient Treatment of Symptomatic, High-Risk Covid-19 Patients that Should be Ramped-Up Immediately as Key to the Pandemic Crisis”, American Journal of Epidemiology 189 (2020), 1218-1226 https://doi.org/10.1093/aje/kwaa093
- H.A. Risch. “Hydroxychloroquine in Early Treatment of High-Risk COVID-19 Outpatients: Efficacy and Safety Evidence.” Sixth version, updated June 17, 2021. https://earlycovidcare.org/wp-content/uploads/2021/09/Evidence-Brief-Risch-v6.pdf
- Prevention and Treatment Protocols for COVID-19. FLCCC Alliance: https://covid19criticalcare.com/covid-19-protocols/
- Bryant A, Lawrie TA, Dowswell T, et al. Ivermectin for Prevention and Treatment of COVID-19 Infection: A Systematic Review, Meta-analysis, and Trial Sequential Analysis to Inform Clinical Guidelines. Am J Ther. 2021;28(4):e434-e460. Published 2021 Jun 21. doi:10.1097/MJT.0000000000001402
- Kory P, Meduri GU, Varon J, Iglesias J, Marik PE. Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19. Am J Ther. 2021 Apr 22;28(3):e299-e318. doi: 10.1097/MJT.0000000000001377. PMID: 34375047; PMCID: PMC8088823.
- Jans DA, Wagstaff KM. The broad-spectrum host-directed agent ivermectin as an antiviral for SARS-CoV-2 ? Biochem Biophys Res Commun. 2021 Jan 29;538:163-172. doi: 10.1016/j.bbrc.2020.10.042. Epub 2020 Oct 21. PMID: 33341233; PMCID: PMC7577703.
- Alexander PE, Armstrong R, Fareed G, et al. Early multidrug treatment of SARS-CoV-2 infection (COVID-19) and reduced mortality among nursing home (or outpatient/ambulatory) residents. Med Hypotheses. 2021;153:110622. doi:10.1016/j.mehy.2021.110622
- Santin AD, Scheim DE, McCullough PA, Yagisawa M, Borody TJ. Ivermectin: a multifaceted drug of Nobel prize-honoured distinction with indicated efficacy against a new global scourge, COVID-19. New Microbes New Infect. 2021;43:100924. Published 2021 Aug 3. doi:10.1016/j.nmni.2021.100924
- McCullough PA, Oskoui R. Early multidrug regimens in new potentially fatal medical problems. Rev Cardiovasc Med. 2020;21(4):507–508.
- Procter BC, Ross C, Pickard V, Smith E, Hanson C, McCullough PA. Clinical outcomes after early ambulatory multidrug therapy for high-risk SARS-CoV-2 (COVID-19) infection. Rev Cardiovasc Med. 2020;21(4):611–614.
- Procter BC, Ross C, Pickard V, Smith E, Hanson C, McCullough PA. Early ambulatory multidrug therapy reduces hospitalization and death in high-risk patients with SARS-CoV-2 (COVID-19) Authorea. January 07, 2021, doi: 10.22541/au.161000355.54720791/v1.
- McCullough PA, Alexander PE, Armstrong R. Multifaceted highly targeted sequential multidrug treatment of early ambulatory high-risk SARS-CoV-2 infection (COVID-19) Rev Cardiovasc Med. 2020;21(4):517–530.
- McCullough PA. Regarding: “Hydroxychloroquine: a comprehensive review and its controversial role in coronavirus disease 2019”. Ann Med. 2021;53(1):286. doi:10.1080/07853890.2021.1872094
- McCullough PA. Favipiravir and the Need for Early Ambulatory Treatment of SARS-CoV-2 Infection (COVID-19). Antimicrob Agents Chemother. 2020;64(12):e02017-20. Published 2020 Nov 17. doi:10.1128/AAC.02017-20
- Derwand R, Scholz M, Zelenko V. COVID-19 outpatients: early risk-stratified treatment with zinc plus low-dose hydroxychloroquine and azithromycin: a retrospective case series study. Int J Antimicrob Agents. 2020;56(6):106214. doi:10.1016/j.ijantimicag.2020.106214
- Cobos-Campos R, Apiñaniz A, Parraza N, Cordero J, García S, Orruño E. Potential use of ivermectin for the treatment and prophylaxis of SARS-CoV-2 infection [published online ahead of print, 2021 Aug 11]. Curr Res Transl Med. 2021;69(4):103309. doi:10.1016/j.retram.2021.103309
- Ngo BT, Marik P, Kory P, et al. The time to offer treatments for COVID-19. Expert Opin Investig Drugs. 2021;30(5):505-518. doi:10.1080/13543784.2021.1901883
- Kory P, Kanne JP. SARS-CoV-2 organising pneumonia: ‘Has there been a widespread failure to identify and treat this prevalent condition in COVID-19?’. BMJ Open Respir Res. 2020;7(1):e000724. doi:10.1136/bmjresp-2020-000724
- Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120-128. doi:10.1056/NEJMoa2015432
- Singhania N, Bansal S, Nimmatoori DP, Ejaz AA, McCullough PA, Singhania G. Current Overview on Hypercoagulability in COVID-19. Am J Cardiovasc Drugs. 2020;20(5):393-403. doi:10.1007/s40256-020-00431-z
- Cavalli E, Bramanti A, Ciurleo R, et.al. Entangling COVID-19 associated thrombosis into a secondary antiphospholipid antibody syndrome: Diagnostic and therapeutic perspectives (Review). International Journal of Molecular Medicine. DOI: 10.3892/ijmm.2020.4659
- Keller MJ, et al. Effects of systemic corticosteroid on mortality or mechanical ventilation in patients with COVID-19. J of Hospital medicine. Published July 22, 2020. Doi:10.12788/jhm.3497
- Kyriazopoulou, E., Poulakou, G., Milionis, H. et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med (2021). https://doi.org/10.1038/s41591-021-01499-z
- Chinese Clinical Trial Registry. A multicenter, randomized controlled trial for the efficacy and safety of tocilizumab in the treatment of new coronavirus pneumonia (COVID-19). Feb 13, 2020. http://www.chictr.org.cn/ showprojen.aspx?proj=49409 (accessed March 6, 2020). doi: 10.1016/S0140-6736(20)30628-0. Epub 2020 Mar 16.
- McGonagle D, Sharif K, O’Regan A, Bridgewood C. Interleukin-6 use in COVID-19 pneumonia related macrophage activation syndrome. Autoimmunity Reviews 2020: 102537. doi: 10.1016/j.autrev.2020.102537
- Toniati P, Piva S, Cattalini M, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev 2020; published online May 3. DOI:10.1016/ j. autrev.2020.102568.
- Zhou W, Liu Y, Tian D, Wang C, Wang S et al. Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal Transduction and Targeted Therapy 2020; 5: 18. doi: 10.1038/s41392-020-0127-9
- Cavalli G, De Luca G, Campochiaro C, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol 2020; 2: e325–31.
- Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol 2020; 2: e393–400.
- Aouba A, Baldolli A, Geffray L, et al. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann Rheum Dis 2020; published online May 6. DOI:10.1136/ annrheumdis-2020-217706.
- Pontali E, Volpi S, Antonucci G, et al. Safety and efficacy of early high-dose IV anakinra in severe COVID-19 lung disease. J Allergy Clin Immunol 2020; published online May 11. DOI:10.1016%2Fj. jaci.2020.05.002.
- Cao W, Liu X, Bai T, Fan H, Hong K et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infectious Diseases. 2020; 7 (3): ofaa102. doi: 10.1093/ ofid/ofaa102
- Conti P, Gallenga CE, Tete G, Caraffa A, Ronconi G et al. How to reduce the likelihood of coronavirus-19 (CoV-19 or SARSCoV-2) infection and lung inflammation mediated by IL-1. Journal of Biological Regulators and Homeostatic Agents 2020; 34 (2). doi: 10.23812/Editorial-Conti-2
- Hung IF. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomized, phase 2 trial. Lancet. 2020; May 10.
- Nile SH, Nile A, Qiu J, et al. COVID-19: Pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020; May 7.
- Shalhoub S. Interferon beta-1b for COVID-19 . Lancet. 2020; May 10.
- Díez JM, Romero C, Gajardo R. Currently available intravenous immunoglobulin contains antibodies reacting against severe acute respiratory syndrome coronavirus 2 antigens. Immunotherapy. 2020; May 13.
- Goursaud S. Corticosteroid use in selected patients with severe acute respiratory distress syndrome related to COVID-19 . J Infect. 2020; May 14.
- Wolhfarth P, Agis H, Gualdoni GA, et al. (2019). Interleukin 1 receptor antagonist anakinra, intravenous immunoglobulin, and corticosteroids in the management of critically ill adult patients with secondary hemophagocytic lymphohistiocytosis. J Intens Care Med. 2019; 34: 723-731.
- Capra R. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur J Intern Med. 2020; May 13.
- Chen P, et al. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with COVID-19. NEJM. October 28, 2020.
- Tseng, C. T., Sbrana, E., Iwata-Yoshikawa, N., Newman, P. C., Garron, T., Atmar, R. L., Peters, C. J., & Couch, R. B. (2012). Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PloS one, 7(4), e35421. https://doi.org/10.1371/journal.pone.0035421
- Haagmans BL, Boudet F, Kuiken T, deLang A, Martina BE, et al. (2005) Protective immunity induced by the inactivated SARS coronavirus vaccine. Abstract S 12-1. Presented at the X International Nidovirus Symposium, Colorado Springs, CO.
- Castilow EM, Olson MR, Varga SM (2007) Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease. Immunol Res 39: 225–239. 33.
- Collins PL, Graham BS (2008) Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol 82: 2040–2055.
- Weiss RC, Scott FW (1981) Antibody-mediated enhancement of disease in feline infectious peritonitis: comparisons with dengue hemorrhagic fever. Comp Immunol Microbiol Infect Dis 4: 175–89.
- Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, et al. (1969) Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 89: 422–34. 42.
- Waris ME, Tsou C, Erdman DD, Zaki SR, Anderson LJ (1996) Respiratory syncytial virus infection in BALB/c mice previously immunized with formalin inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J Virol 70: 2852–60. 43.
- Polack FP, Teng MN, Collins PL, Prince GA, Exner M, et al. (2002) A role for immune complexes in enhanced respiratory syncytial virus disease. J Exp Med 196: 859–65.
- Lee, W.S., Wheatley, A.K., Kent, S.J. et al. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat Microbiol 5, 1185–1191 (2020). https://doi.org/10.1038/s41564-020-00789-5
- Lyons-Weiler J. Pathogenic priming likely contributes to serious and critical illness and mortality in COVID-19 via autoimmunity. Journal of Translational Autoimmunity 3 (2020) 100051. https://doi.org/10.1016/j.jtauto.2020.100051
- Yahi N, Chahinian H, Fantini J. Infection-enhancing anti-SARS-CoV-2 antibodies recognize both the original Wuhan / D614G strain and Delta variants. A potential risk for mass vaccination? J Infect. 2021 Aug 9: S0163-4453 (21) 00392-3.
- Ulrich H, Pillat MM, Tárnok A. Dengue Fever, COVID-19 (SARS-CoV-2), and Antibody-Dependent Enhancement (ADE): A Perspective. Cytometry A. 2020 Jul; 97 (7): 662-667.
- Cardozo T, Veazey R. Informed consent disclosure to vaccine trial subjects of risk of COVID-19 vaccines worsening clinical disease. Int J Clin Pract. 2021 Mar; 75 (3): e13795.
- Hohdatsu, T. et al. Antibody-dependent enhancement of feline infectious peritonitis virus infection in feline alveolar macrophages and human monocyte cell line U937 by serum of cats experimentally or naturally infected with feline coronavirus. J. Vet. Med. Sci. 60, 49–55 (1998).
- Halstead, S. B. & O’Rourke, E. J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J. Exp. Med. 146, 201–217 (1977).
- Vennema, H. et al. Early death after feline infectious peritonitis virus challenge due to recombinant vaccinia virus immunization. J. Virol. 64, 1407–1409 (1990).
- Hohdatsu, T., Nakamura, M., Ishizuka, Y., Yamada, H. & Koyama, H. A study on the mechanism of antibody-dependent enhancement of feline infectious peritonitis virus infection in feline macrophages by monoclonal antibodies. Arch. Virol. 120, 207–217 (1991).
- Ye, Z. W. et al. Antibody-dependent cell-mediated cytotoxicity epitopes on the hemagglutinin head region of pandemic H1N1 influenza virus play detrimental roles in H1N1-infected mice. Front. Immunol. 8, 317 (2017).
- Winarski, K. L. et al. Antibody-dependent enhancement of influenza disease promoted by increase in hemagglutinin stem flexibility and virus fusion kinetics. Proc. Natl Acad. Sci. USA 116, 15194–15199 (2019).
- Gao, T. et al. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. Preprint at https://www.medrxiv.org/content/10.1101/2020.03.29.20041962v3 (2020).
- Gralinski, L. E. et al. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio 9, e01753-18 (2018).
- Wang, T. T. et al. IgG antibodies to dengue enhanced for FcγRIIIA binding determine disease severity. Science 355, 395–398 (2017).
- Yip, M. S. et al. Antibody-dependent infection of human macrophages by severe acute respiratory syndrome coronavirus. Virol. J. 11, 82 (2014).
- Takada, A., Watanabe, S., Okazaki, K., Kida, H. & Kawaoka, Y. Infectivity-enhancing antibodies to Ebola virus glycoprotein. J. Virol. 75, 2324–2330 (2001).
- Takada, A., Feldmann, H., Ksiazek, T. G. & Kawaoka, Y. Antibody-dependent enhancement of Ebola virus infection. J. Virol. 77, 7539–7544 (2003).
- Ochiai, H. et al. Infection enhancement of influenza A NWS virus in primary murine macrophages by anti-hemagglutinin monoclonal antibody. J. Med. Virol. 36, 217–221 (1992).
- Wan, Y. et al. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J. Virol. 94, e02015-19 (2020).
- Jaume, M. et al. Anti-severe acute respiratory syndrome coronavirus spike antibodies trigger infection of human immune cells via a pH- and cysteine protease-independent FcγR pathway. J. Virol. 85, 10582–10597 (2011).
- Cheung, C. Y. et al. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J. Virol. 79, 7819–7826 (2005).
- Yip, M. S. et al. Antibody-dependent enhancement of SARS coronavirus infection and its role in the pathogenesis of SARS. Hong Kong Med. J. 22, 25–31 (2016).
- Ana-Sosa-Batiz, F. et al. Influenza-specific antibody-dependent phagocytosis. PLoS ONE 11, e0154461 (2016).
- Bolles, M. et al. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J. Virol. 85, 12201–12215 (2011).
- Yasui, F. et al. Prior immunization with severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV) nucleocapsid protein causes severe pneumonia in mice infected with SARS-CoV. J. Immunol. 181, 6337–6348 (2008).
- Agrawal, A. S. et al. Immunization with inactivated Middle East respiratory syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum. Vaccin. Immunother. 12, 2351–2356 (2016).
- Weingartl, H. et al. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J. Virol. 78, 12672–12676 (2004).
- Liu, L. et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight 4, e123158 (2019).
- Luo, F. et al. Evaluation of antibody-dependent enhancement of SARS-CoV infection in rhesus macaques immunized with an inactivated SARS-CoV vaccine. Virol. Sin. 33, 201–204 (2018).
- Dolton G, et al. Emergence of immune escape at dominant SARS-CoV-2 killer T-cell epitope. medRxiv preprint doi: https://doi.org/10.1101/2021.06.21.21259010
- Martin DP, et al. (2021). The emergence and ongoing convergent evolution of the N501Y lineages coincides with a major global shift in the SARS-CoV-2 selective landscape. medRxiv : the preprint server for health sciences, 2021.02.23.21252268. https://doi.org/10.1101/2021.02.23.21252268
- Van Egeren D, et al. (2021) Risk of rapid evolutionary escape from biomedical interventions targeting SARS-CoV-2 spike protein. PLOS ONE 16(4): e0250780. https://doi.org/10.1371/journal.pone.0250780
- Harvey WT, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nature Reviews/Microbiology. Vol 19, July 2021, 409. https://www.nature.com/articles/s41579-021-00573-0.pdf
- Restif O, Grenfell BT. Vaccination and the dynamics of immune evasion. J R Soc Interface. 2007 Feb 22;4(12):143-53. doi: 10.1098/rsif.2006.0167. PMID: 17210532; PMCID: PMC2358969.
- Zhou D, et al., Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell 189, 2348–2361 April 29, 2021. https://doi.org/10.1016/j.cell.2021.02.037
- Read AF, Baigent SJ, Powers C, Kgosana LB, Blackwell L, et al. (2015) Imperfect Vaccination Can Enhance the Transmission of Highly Virulent Pathogens. PLOS Biology 13(7): e1002198. https://doi.org/10.1371/journal.pbio.1002198
- Atlani-Duault L, et al. Immune evasion means we need a new COVID-19 social contract. The Lancet Public Health. Vol 6, issue 4, E199-E200, April 1, 2021. https://doi.org/10.1016/ S2468-2667(21)00036-0
- Karim SSA. Vaccines and SARS-CoV-2 variants: the urgent need for a correlate of protection. The Lancet; March 22, 2021. https://doi.org/10.1016/S0140-6736(21)00468-2
- Liu Y, et al. The SARS-CoV-2 Delta variant is poised to acquire complete resistance to wild-type spike vaccines. bioRxiv preprint doi: https://doi.org/10.1101/2021.08.22.457114
- A report of the Chief Science Advisor of Canada, July 16, 2021: COVID-19 vaccine-associated myocarditis/pericarditis: https://science.gc.ca/eic/site/063.nsf/eng/h_98291.html
- Abbate, et al. Fulminant myocarditis and systemic hyper inflammation temporally associated with BNT162b2 COVID-19 mRNA vaccination in two patients: https://www.sciencedirect.com/science/article/pii/S0167527321012286.
- Abbattista, et al. Comparison of adverse drug reactions among four COVID-19 vaccines in Europe using the EudraVigilance database: Thrombosis in unusual sites: https://pubmed.ncbi.nlm.nih.gov/34375510/
- Abe Zeid Daou, et al. Biphasic anaphylaxis after exposure to the first dose of the Pfizer-BioNTech COVID-19 mRNA vaccine COVID-19: https://pubmed.ncbi.nlm.nih.gov/34050949/
- Abicic, et al. Miller Fisher syndrome after Pfizer COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34817727/.
- Abou-Foul, et al. Cervical lymphadenopathy after coronavirus disease vaccination 2019: clinical features and implications for head and neck cancer services: https://pubmed.ncbi.nlm.nih.gov/34526175/
- Abou-Ismail, et al. Vaccine-induced thrombotic thrombocytopenia after Ad26.COV2.S vaccination in a man presenting as acute venous thromboembolism: https://pubmed.ncbi.nlm.nih.gov/34096082/
- Ahmad, et al. Post COVID-19 transverse myelitis; a case report with review of the literature: https://pubmed.ncbi.nlm.nih.gov/34457267/.
- Ai S, et al. Hemophagocytic lymphohistiocytosis after vaccination with ChAdOx1 nCov-19: https://pubmed.ncbi.nlm.nih.gov/34406660/.
- Akiyama, et al. Immune thrombocytopenia associated with the Pfizer-BioNTech COVID-19 mRNA vaccine BNT162b2: https://www.sciencedirect.com/science/article/pii/S2214250921002018
- Aladdin, et al. New-onset refractory status epilepticus after ChAdOx1 nCoV-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34153802/
- Aladdin, et al. Vaccine-induced immune thrombotic thrombocytopenia with disseminated intravascular coagulation and death after ChAdOx1 nCoV-19 vaccination: https://www.sciencedirect.com/science/article/pii/S1052305721003414
- Alalwan, et al. COVID-19 vaccine-induced thrombotic thrombocytopenia: a case series: https://pubmed.ncbi.nlm.nih.gov/34527501/
- Alania-Torres, et al. Case report: probable myocarditis after Covid-19 mRNA vaccine in a patient with arrhythmogenic left ventricular cardiomyopathy: https://pubmed.ncbi.nlm.nih.gov/34712717/.
- Albert, E., Aurigemma, G., Saucedo, J., & Gerson, D. S. (2021). Myocarditis following COVID-19 vaccination. Radiol Case Rep, 16(8), 2142-2145. doi:10.1016/j.radcr.2021.05.033. https://www.ncbi.nlm.nih.gov/pubmed/34025885
- Aleem, et al. Coronavirus (COVID-19) Vaccine-induced immune thrombotic thrombocytopenia (VITT): https://pubmed.ncbi.nlm.nih.gov/34033367/
- Allen, et al. Variant Guillain-Barré syndrome occurring after SARS-CoV-2 vaccination: https://pubmed.ncbi.nlm.nih.gov/34114269/.
- Al-Maqbali, et al. 59-year-old woman with extensive deep venous thrombosis and pulmonary thromboembolism 7 days after a first dose of Pfizer-BioNTech BNT162b2 mRNA vaccine COVID-19: https://pubmed.ncbi.nlm.nih.gov/34117206/
- Al-Mashdali, et al. Post COVID-19 vaccine acute hyperactive encephalopathy with dramatic response to methylprednisolone: a case report: https://www.sciencedirect.com/science/article/pii/S2049080121007536
- Al-Mayhani, et al. Ischemic stroke as a presenting feature of immune thrombotic thrombocytopenia induced by ChAdOx1-nCoV-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34035134/
- Alnaes, et al. COVID-19 vaccines increase the risk of anaphylaxis: https://pubmed.ncbi.nlm.nih.gov/33685103/
- Al-Samkari, et al. Transient thrombocytopenia with glycoprotein-specific platelet autoantibodies after vaccination with Ad26.COV2.S: case report: https://pubmed.ncbi.nlm.nih.gov/34516272/.
- Althaus, et al. Procoagulant antibody-mediated procoagulant platelets in immune thrombotic thrombocytopenia associated with SARS-CoV-2 vaccination: https://pubmed.ncbi.nlm.nih.gov/34011137/.
- Ambati, et al. Acute myopericarditis after COVID-19 vaccination in adolescents: https://pubmed.ncbi.nlm.nih.gov/34589238/.
- Ambrosetti, et al. COVID-19 vaccine-induced thrombosis: https://pubmed.ncbi.nlm.nih.gov/34802488/.
- Ammirati, et al. Temporal relation between the second dose of BNT162b2 mRNA Covid-19 vaccine and cardiac involvement in a patient with previous SARS-COV-2 infection: https://www.sciencedirect.com/science/article/pii/S2352906721000622
- An et al. Reactive arthritis after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34033732/.
- Anderson, et al. Occurrence of splenic infarction due to arterial thrombosis after vaccination with COVID-19: https://pubmed.ncbi.nlm.nih.gov/34876440/
- Andraska, et al. Three cases of acute venous thromboembolism in women after coronavirus 2019 vaccination: https://pubmed.ncbi.nlm.nih.gov/34352418/
- Angeli F, Spanevello A, Reboldi G, Visca D, Verdecchia P. SARS-CoV-2 vaccines: Lights and shadows. Eur J Intern Med. 2021 Jun; 88: 1-8.
- Annabi, et al. Rare cutaneous adverse effects of COVID-19 vaccines: a case series and review of the literature: https://pubmed.ncbi.nlm.nih.gov/34363637/
- Anupama, et al. Nephrotic syndrome after ChAdOx1 nCoV-19 vaccine against SARScoV-2: https://pubmed.ncbi.nlm.nih.gov/34250318/.
- Aomar-Millan, et al. COVID-19, Guillain-Barré and vaccine: A dangerous combination: https://pubmed.ncbi.nlm.nih.gov/34108736/.
- Arcolaci, et al. Immunization practices and risk of anaphylaxis: a current, comprehensive update of COVID-19 vaccination data: https://pubmed.ncbi.nlm.nih.gov/34269740/
- Ardalan, et al. Shingles-like skin lesion after vaccination with AstraZeneca for COVID-19: a case report: https://pubmed.ncbi.nlm.nih.gov/34631069/
- Armoni-Weiss, et al. Hailey-Hailey disease exacerbation after SARS-CoV-2 vaccination: https://pubmed.ncbi.nlm.nih.gov/34436620/
- Arola, A., et al., Occurrence and Features of Childhood Myocarditis: A Nationwide Study in Finland. J Am Heart Assoc, 2017. 6(11).
- Aroliga, et al. Allergic reactions and adverse events associated with administration of mRNA-based vaccines. A health system experience: https://pubmed.ncbi.nlm.nih.gov/34474708/
- Ashaari, et al. Case report: symptomatic pericarditis post COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34693198/.
- Ashoor, et al. Axillary lymphadenopathy in patients with recent Covid-19 vaccination: a new diagnostic dilemma: https://pubmed.ncbi.nlm.nih.gov/34825530/.
- Ashrani, et al. Age- and sex-specific incidence of cerebral venous sinus thrombosis associated with Ad26.COV2.S COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34724036/.
- Asmat, et al. A rare case of COVID-19 vaccine-induced thrombotic thrombocytopenia (VITT) affecting the venosplanchnic and pulmonary arterial circulation from a UK district general hospital: https://pubmed.ncbi.nlm.nih.gov/34535492/
- Aye, Y. N., Mai, A. S., Zhang, A., Lim, O. Z. H., Lin, N., Ng, C. H., . . . Chew, N. W. S. (2021). Acute Myocardial Infarction and Myocarditis following COVID-19 Vaccination. QJM. doi:10.1093/qjmed/hcab252. https://www.ncbi.nlm.nih.gov/pubmed/34586408
- Azir, et al. STEMI mimicry: focal myocarditis in an adolescent patient after COVID-19 mRNA vaccination: https://pubmed.ncbi.nlm.nih.gov/34756746/
- Badier, et al. IgA vasculitis in adult patient after vaccination with ChadOx1 nCoV-19: https://pubmed.ncbi.nlm.nih.gov/34509658/
- Badoiu, et al. Clinical variant of Guillain-Barré syndrome with prominent facial diplegia after AstraZeneca 2019 coronavirus disease vaccine: https://pubmed.ncbi.nlm.nih.gov/34808658/
- Baldi, et al. Thrombotic events after COVID-19 vaccination in over 50 years of age: results of a population-based study in Italy: https://pubmed.ncbi.nlm.nih.gov/34835237/
- Bandapaati, et al. celiac artery and splenic artery thrombosis complicated by splenic infarction 7 days after the first dose of Oxford vaccine, causal relationship or coincidence: https://pubmed.ncbi.nlm.nih.gov/34261633/.
- Banerjee, et al. Immune-mediated thrombocytopenia associated with Ad26.COV2.S vaccine (Janssen; Johnson & Johnson): https://pubmed.ncbi.nlm.nih.gov/34469919/.
- Barda, N., Dagan, N., Ben-Shlomo, Y., Kepten, E., Waxman, J., Ohana, R., . . . Balicer, R. D. (2021). Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N Engl J Med, 385(12), 1078-1090. doi:10.1056/NEJMoa2110475. https://www.ncbi.nlm.nih.gov/pubmed/34432976
- Bari, et al. COVID-19: lessons from the Norwegian tragedy should be taken into account in planning for vaccine launch in less developed/developing countries: https://pubmed.ncbi.nlm.nih.gov/34435142/
- Barral, et al. Thromboaspiration infusion and fibrinolysis for portomesenteric thrombosis after administration of AstraZeneca COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34132839/
- Barsha, et al. A case of acute encephalopathy and non-ST-segment elevation myocardial infarction after vaccination with mRNA-1273: possible adverse effect: https://pubmed.ncbi.nlm.nih.gov/34703815/
- Bayas, et al. Bilateral superior ophthalmic vein thrombosis, ischemic stroke and immune thrombocytopenia after vaccination with ChAdOx1 nCoV-19: https://pubmed.ncbi.nlm.nih.gov/33864750/
- Ben David, et al. Rate of recurrent Guillain-Barré syndrome after COVID-19 BNT162b2 mRNA vaccine: https://jamanetwork.com/journals/jamaneurology/fullarticle/2783708
- Bencharattanaphakhi, et al. Cutaneous leukocytoclastic vasculitis induced by Sinovac COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34660867/.
- Bennett, et al. Newly diagnosed immune thrombocytopenia in a pregnant patient after coronavirus disease 2019 vaccination: https://pubmed.ncbi.nlm.nih.gov/34420249/
- Berry, et al. Cutaneous small-vessel vasculitis after vaccination with a single dose of Janssen Ad26.COV2.S: https://pubmed.ncbi.nlm.nih.gov/34337124/
- Berto, et al. Takotsubo cardiomyopathy after vaccination with mRNA COVID-19: https://www.sciencedirect.com/science/article/pii/S1443950621011331
- Bhan, et al. An unusual presentation of acute deep vein thrombosis after Modern COVID-19 vaccine: case report: https://pubmed.ncbi.nlm.nih.gov/34790811/
- Bhandari, M., Pradhan, A., Vishwakarma, P., & Sethi, R. (2021). Coronavirus and cardiovascular manifestations- getting to the heart of the matter. World J Cardiol, 13(10), 556-565. doi:10.4330/wjc.v13.i10.556. https://www.ncbi.nlm.nih.gov/pubmed/34754400
- Biancatelli RMLC, et al. The SARS-CoV-2 spike protein subunit S1 induces COVID-19-like acute lung injury in K18-hACE2 transgenic mice and barrier dysfunction in human endothelial cells Am J Physiol Lung Cell Mol Physiol 321: L477–L484, 2021
- Bikdeli, et al. Cerebral venous sinus thrombosis in the U.S. population, After SARS-CoV-2 vaccination with adenovirus and after COVID-19: https://pubmed.ncbi.nlm.nih.gov/34116145/
- Bin Waqar, et al. Thrombotic thrombocytopenic purpura: a new threat after COVID bnt162b2 vaccine: https://pubmed.ncbi.nlm.nih.gov/34264514/.
- Bjornstad-Tuveng, et al. Fatal cerebral hemorrhage after COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/33928772/
- Blauenfeldt, et al. Thrombocytopenia with acute ischemic stroke and hemorrhage in a patient recently vaccinated with an adenoviral vector-based COVID-19 vaccine:. https://pubmed.ncbi.nlm.nih.gov/33877737/
- Blumenthal, et al. Acute allergic reactions to COVID-19 mRNA vaccines: https://pubmed.ncbi.nlm.nih.gov/33683290/
- Bogdanov, et al. Cutaneous adverse effects of available COVID-19 vaccines: https://pubmed.ncbi.nlm.nih.gov/34518015/
- Boivin, et al. Premature myocardial infarction or side effect of COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/33824804/
- Bonato, et al. Massive cerebral venous thrombosis due to vaccine-induced immune thrombotic thrombocytopenia: https://pubmed.ncbi.nlm.nih.gov/34261296/
- Bonifacio, et al. Bilateral facial weakness with a variant of paresthesia of Guillain-Barre syndrome after Vaxzevria COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34261746/
- Book, et al. Bilateral acute macular neuroretinopathy after SARS-CoV-2 vaccination: https://pubmed.ncbi.nlm.nih.gov/34287612/
- Bostan, et al. Exacerbation of plaque psoriasis after COVID-19 inactivated mRNA and BNT162b2 vaccines: report of two cases: https://pubmed.ncbi.nlm.nih.gov/34427024/
- Bouattour, et al. Guillain-Barré syndrome after the first dose of Pfizer-BioNTech COVID-19 vaccine: case report and review of reported cases: https://pubmed.ncbi.nlm.nih.gov/34796417/.
- Bourguignon, et al. Adjunct Immunoglobulin for vaccine-induced immune thrombotic thrombocytopenia: https://pubmed.ncbi.nlm.nih.gov/34107198/
- Boursier, et al. Ga-DOTATOC digital PET images of inflammatory cell infiltrates in myocarditis after vaccination with COVID-19: https://pubmed.ncbi.nlm.nih.gov/34746968/
- Bozkurt, et al. Myocarditis with COVID-19 mRNA vaccines: https://www.ahajournals.org/doi/pdf/10.1161/CIRCULATIONAHA.121.056135
- Braun, et al. Case report: Take a second look: Cerebral venous thrombosis related to Covid-19 vaccination and thrombotic thrombocytopenia syndrome: https://pubmed.ncbi.nlm.nih.gov/34880826/
- Bril, et al. Autoimmune hepatitis developing after coronavirus disease vaccine 2019 (COVID-19): causality or victim: https://pubmed.ncbi.nlm.nih.gov/33862041/
- Buchan, et al. Epidemiology of myocarditis and pericarditis following mRNA vaccines in Ontario, Canada: by vaccine product, schedule, and interval: https://www.medrxiv.org/content/10.1101/2021.12.02.21267156v1
- Buchhorn, R., Meyer, C., Schulze-Forster, K., Junker, J., & Heidecke, H. (2021). Autoantibody Release in Children after Corona Virus mRNA Vaccination: A Risk Factor of Multisystem Inflammatory Syndrome? Vaccines (Basel), 9(11). doi:10.3390/vaccines9111353. https://www.ncbi.nlm.nih.gov/pubmed/34835284
- Burillo, et al. Amyotrophic Neuralgia secondary to Vaxzevri vaccine (AstraZeneca) COVID-19: https://pubmed.ncbi.nlm.nih.gov/34330677/
- Burrows, et al. Sequential contralateral facial nerve palsy after first and second doses of COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34281950/.
- Buzhdygana, TP, DeOrec, BJ, Baldwin-Leclair, A., Bullock, TA, McGary, H. M, Ramirez, SH (2020). The SARS-CoV-2 Spike Protein Alters Barrier Function in 2D Static and 3D Microfluidic in-Vitro Models of the Human Blood-Brain Barrier. Neurobiology of Disease 146: 105131.
- Cabanillas, et al. Allergic reactions to the first COVID-19 vaccine: a potential role of polyethylene glycol: https://pubmed.ncbi.nlm.nih.gov/33320974/
- Calcaterra, et al. COVID 19 vaccine for adolescents. Concern for myocarditis and pericarditis: https://www.mdpi.com/2036-7503/13/3/61.
- Calcaterra, G., Bassareo, P. P., Barilla, F., Romeo, F., & Mehta, J. L. (2022). Concerning the unexpected prothrombotic state following some coronavirus disease 2019 vaccines. J Cardiovasc Med (Hagerstown), 23(2), 71-74. doi:10.2459/JCM.0000000000001232. https://www.ncbi.nlm.nih.gov/pubmed/34366403
- Cardoso, et al. A case of cervical lymphadenopathy following COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34141500/
- Cari, et al. Blood clots and bleeding after BNT162b2 and ChAdOx1 nCoV-19 vaccination: an analysis of European data: https://pubmed.ncbi.nlm.nih.gov/34174723/
- Carli, et al. Deep venous thrombosis (DVT) occurring shortly after second dose of SARS-CoV-2 mRNA vaccine: https://pubmed.ncbi.nlm.nih.gov/33687691/
- Castelli, et al. Cerebral venous sinus thrombosis associated with thrombocytopenia after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/33845870/.
- Catala, et al. Skin reactions after vaccination against SARS-COV-2: a nationwide Spanish cross-sectional study of 405 cases: https://pubmed.ncbi.nlm.nih.gov/34254291/
- Cattaneo, et al. Thrombosis with thrombocytopenia syndrome associated with COVID-19 viral vector vaccines: https://pubmed.ncbi.nlm.nih.gov/34092488/
- Cavalli, et al. Cutaneous vasculitis following COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34611627/.
- CDC COVID-19 Response Team: Allergic reactions, including anaphylaxis, after receiving the first dose of Pfizer-BioNTech COVID-19 vaccine – United States, December 14-23, 2020: https://pubmed.ncbi.nlm.nih.gov/33444297/
- CDC COVID-19 Response Team: Allergic reactions, including anaphylaxis, after receiving first dose of Modern COVID-19 vaccine – United States, December 21, 2020-January 10, 2021: https://pubmed.ncbi.nlm.nih.gov/33507892/
- CDC Report, September 2, 2021: Selected Adverse Events Reported after COVID-19 Vaccination. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html
- Cebeci, et al. Petechial rash associated with CoronaVac vaccination: first report of cutaneous side effects before phase 3 results: https://ejhp.bmj.com/content/early/2021/05/23/ejhpharm-2021-002794
- Cellina, et al. Left Bell’s palsy after the first dose of mRNA-1273 SARS-CoV-2 vaccine: case report: https://pubmed.ncbi.nlm.nih.gov/34763263/.
- Censcak, et al. Guillain-Barre syndrome after the first dose of COVID-19 vaccination: case report; https://pubmed.ncbi.nlm.nih.gov/34779385/.
- Cereda, et al. Acute myocarditis after the second dose of SARS-CoV-2 vaccine: serendipity or causal relationship: https://pubmed.ncbi.nlm.nih.gov/34236331/
- Ceschia, et al. Diffuse prothrombotic syndrome after administration of ChAdOx1 nCoV-19 vaccine: case report: https://pubmed.ncbi.nlm.nih.gov/34615534/
- Chai, Q., Nygaard, U., Schmidt, R. C., Zaremba, T., Moller, A. M., & Thorvig, C. M. (2022). Multisystem inflammatory syndrome in a male adolescent after his second Pfizer-BioNTech COVID-19 vaccine. Acta Paediatr, 111(1), 125-127. doi:10.1111/apa.16141. https://www.ncbi.nlm.nih.gov/pubmed/34617315
- Chamling, et al. Occurrence of acute infarct-like myocarditis after COVID-19 vaccination: just an accidental coincidence or rather a vaccination-associated autoimmune myocarditis? https://pubmed.ncbi.nlm.nih.gov/34333695/
- Chang JC, et al. Vaccine-associated thrombocytopenia and thrombosis: venous endotheliopathy leading to combined venous micro-macrothrombosis: https://pubmed.ncbi.nlm.nih.gov/34833382/
- Chelala, et al. Myocarditis findings on cardiac magnetic resonance imaging after vaccination with COVID-19 mRNA in adolescents: https://pubmed.ncbi.nlm.nih.gov/34704459/
- Chen VM, et al. Australian and New Zealand approach to the diagnosis and treatment of vaccine-induced immune thrombosis and immune thrombocytopenia: https://pubmed.ncbi.nlm.nih.gov/34490632/
- Chen YA, et al. Palmar digital vein thrombosis after Oxford-AstraZeneca COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34473841/.
- Chen, et al. Beware of neuromyelitis optica spectrum disorder after vaccination with inactivated virus for COVID-19: https://pubmed.ncbi.nlm.nih.gov/34189662/
- Chen, et al. Case report: anti-neutrophil cytoplasmic antibody-associated vasculitis with acute renal failure and pulmonary hemorrhage can occur after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34859017/
- Chen, et al. Nerve and muscle adverse events after vaccination with COVID-19: a systematic review and meta-analysis of clinical trials: https://pubmed.ncbi.nlm.nih.gov/34452064/.
- Cheng N. Cerebral venous sinus thrombosis following vaccination with Pfizer-BioNTech COVID-19 (BNT162b2): https://pubmed.ncbi.nlm.nih.gov/34595867/
- Chiang, CY, et al. Myocardial infarction and azygos vein thrombosis after vaccination with ChAdOx1 nCoV-19 in a hemodialysis patient: https://pubmed.ncbi.nlm.nih.gov/34650896/
- Choi GJ, et al. Fatal systemic capillary leak syndrome after SARS-COV-2 vaccination in a patient with multiple myeloma: https://pubmed.ncbi.nlm.nih.gov/34459725/
- Choi PY, et al. Immune thrombocytopenia after vaccination during the COVID-19 pandemic: https://pubmed.ncbi.nlm.nih.gov/34435486/
- Choi YK, et al. Post-vaccination multisystem inflammatory syndrome in adults without evidence of prior SARS-CoV-2 infection: https://pubmed.ncbi.nlm.nih.gov/34852213/
- Choi, et al. Intracerebral hemorrhage due to thrombosis with thrombocytopenia syndrome after COVID-19 vaccination: the first fatal case in Korea: https://pubmed.ncbi.nlm.nih.gov/34402235/
- Choi, et al. Myocarditis-induced sudden death after BNT162b2 COVID-19 mRNA vaccination in Korea: case report focusing on histopathological findings: https://pubmed.ncbi.nlm.nih.gov/34664804/
- Chouchana, L., Blet, A., Al-Khalaf, M., Kafil, T. S., Nair, G., Robblee, J., . . . Liu, P. P. (2021). Features of Inflammatory Heart Reactions Following mRNA COVID-19 Vaccination at a Global Level. Clin Pharmacol Ther. doi:10.1002/cpt.2499. https://www.ncbi.nlm.nih.gov/pubmed/34860360
- Christensen, et al. Guillian Barré syndrome after vaccination with mRNA-1273 against COVID-19: https://pubmed.ncbi.nlm.nih.gov/34477091/
- Chua, et al. Epidemiology of acute myocarditis/pericarditis in Hong Kong adolescents after co-vaccination: https://academic.oup.com/cid/advance-article-abstract/doi/10.1093/cid/ciab989/6445179
- Chuang TY, et al. Tolosa-Hunt syndrome occurring after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34513398/
- Ciccone, et al. The importance of recognizing cerebral venous thrombosis following anti-COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34001390/
- Ciconne, A. Cerebral venous thrombosis induced by SARS-CoV-2 vaccine: https://pubmed.ncbi.nlm.nih.gov/34090750/.
- Cimaglia, et al. Acute myocarditis after SARS-CoV-2 vaccination in a 24-year-old man: https://www.sciencedirect.com/science/article/pii/S0870255121003243
- Cines, et al. SARS-CoV-2 vaccine-induced thrombotic thrombocytopenia: https://www.nejm.org/doi/full/10.1056/nejme2106315
- Cinotti, et al. Eosinophilic dermatosis after AstraZeneca COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34753210/.
- Cirillo N. Reported orofacial adverse effects from COVID-19 vaccines: the known and the unknown: https://pubmed.ncbi.nlm.nih.gov/33527524/
- Cirillo, et al. Bell’s palsy and SARS-CoV-2 vaccines: an unfolding story: https://www.sciencedirect.com/science/article/pii/S1473309921002735
- Cirillo, et al. The association between COVID-19 vaccination and Bell’s palsy: https://pubmed.ncbi.nlm.nih.gov/34411533/
- Clark, et al. Early outcomes of bivalirudin treatment for thrombotic thrombocytopenia and cerebral venous sinus thrombosis after vaccination with Ad26.COV2.S: https://www.sciencedirect.com/science/article/pii/S0196064421003425
- Clarke, R., & Ioannou, A. (2021). Should T2 mapping be used in cases of recurrent myocarditis to differentiate between the acute inflammation and chronic scar? J Pediatr. doi:10.1016/j.jpeds.2021.12.026. https://www.ncbi.nlm.nih.gov/pubmed/34933012
- Clayton-Chubb, et al. Autoimmune hepatitis developing after ChAdOx1 nCoV-19 vaccine (Oxford-AstraZeneca): https://pubmed.ncbi.nlm.nih.gov/34171435/
- Cleaver, et al. Endovascular treatment for vaccine-induced cerebral venous sinus thrombosis and thrombocytopenia after vaccination with ChAdOx1 nCoV-19: report of three cases: https://pubmed.ncbi.nlm.nih.gov/34782400/
- Cohen, et al. Hypermetabolic lymphadenopathy after administration of BNT162b2 mRNA vaccine Covid-19: incidence assessed by [ 18 F] FDG PET-CT and relevance for study interpretation: https://pubmed.ncbi.nlm.nih.gov/33774684/
- Cohen, et al. Outbreak of leukocytoclastic vasculitis after COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/33928638/
- Colaneri, M., De Filippo, M., Licari, A., Marseglia, A., Maiocchi, L., Ricciardi, A., . . . Bruno, R. (2021). COVID vaccination and asthma exacerbation: might there be a link? Int J Infect Dis, 112, 243-246. doi:10.1016/j.ijid.2021.09.026. https://www.ncbi.nlm.nih.gov/pubmed/34547487
- Colella, et al. Bell’s palsy after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/33611630/
- Colia, et al. Cutaneous vasculitis after severe acute respiratory syndrome coronavirus 2 vaccine: https://pubmed.ncbi.nlm.nih.gov/34557622/.
- Conticini, et al. Relapse of microscopic polyangiitis after COVID-19 vaccination: case report: https://pubmed.ncbi.nlm.nih.gov/34251683/.
- Cooper, et al. Severe immune thrombocytopenic purpura after SARS-CoV-2 vaccine: https://pubmed.ncbi.nlm.nih.gov/34754937/
- Correa, eta l. Neurological symptoms and neuroimaging alterations related to COVID-19 vaccine: cause or coincidence: https://www.sciencedirect.com/science/article/pii/S0899707121003557.
- Cory, et al. Hemophagocytic lymphohistiocytosis following COVID-19 vaccination (ChAdOx1 nCoV-19): https://pubmed.ncbi.nlm.nih.gov/34862234/
- Costentin, et al. Acute ischemic stroke revealing immune thrombotic thrombocytopenia induced by ChAdOx1 nCov-19 vaccine: impact on recanalization strategy: https://pubmed.ncbi.nlm.nih.gov/34175640/
- Coto-Seguta, et al. Vesiculobullous cutaneous reactions induced by COVID-19 mRNA vaccine: report of four cases and review of the literature: https://pubmed.ncbi.nlm.nih.gov/34236711/
- COVID-19 RNA-based vaccines and the risk of prion disease: https://scivisionpub.com/pdfs/covid19rna-based-vaccines-and-the-risk-of-prion-dis ease-1503.pdf
- Crane, et al. Takotsubo (stress) cardiomyopathy after vaccination with ChAdOx1 nCoV-19: https://pubmed.ncbi.nlm.nih.gov/34625447/
- Crea F. Thrombosis in peripheral artery disease and thrombotic thrombocytopenia following adenoviral COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34649281/.
- Cristofaro, et al. Vaccine-induced thrombotic thrombocytopenia, a rare but severe case of friendly fire in the battle against the COVID-19 pandemic: What pathogenesis?: https://www.sciencedirect.com/science/article/pii/S0953620521002314
- Cugno, et al. Increased risk of urticaria/angioedema after BNT162b2 mRNA COVID-19 vaccination in health care workers taking ACE inhibitors: https://pubmed.ncbi.nlm.nih.gov/34579248/
- Cuker A. Thrombosis with thrombocytopenia syndrome after COVID-19 immunization: https://pubmed.ncbi.nlm.nih.gov/34236343/
- D’ Agostino, et al. A rare case of cerebral venous thrombosis and disseminated intravascular coagulation temporally associated with administration of COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/33917902/
- D’ Angelo, et al. Myocarditis after SARS-CoV-2 vaccination: a vaccine-induced reaction: https://pubmed.ncbi.nlm.nih.gov/34118375/
- Dalan, et al. Thrombosis after COVID-19 vaccination: possible link to ACE pathways: https://www.sciencedirect.com/science/article/pii/S0049384821004369
- Dalwadi, et al. Guillian-Barre syndrome with axonal variant temporally associated with Modern SARS-CoV-2 mRNA-based vaccine: https://pubmed.ncbi.nlm.nih.gov/34722067/
- Dang, et al. Miller-Fisher syndrome and Guillain-Barré syndrome overlap syndrome in a patient after Oxford-AstraZeneca SARS-CoV-2 vaccination: https://pubmed.ncbi.nlm.nih.gov/34848426/.
- Daou, et al. Biphasic anaphylaxis after exposure to the first dose of Pfizer-BioNTech COVID-19 mRNA vaccine COVID-19: https://pubmed.ncbi.nlm.nih.gov/34050949/
- Das, B. B., Moskowitz, W. B., Taylor, M. B., & Palmer, A. (2021). Myocarditis and Pericarditis Following mRNA COVID-19 Vaccination: What Do We Know So Far? Children (Basel), 8(7). doi:10.3390/children8070607. https://www.ncbi.nlm.nih.gov/pubmed/34356586
- Das, et al. Myopericarditis after COVID-19 mRNA vaccination in adolescents 12 to 18 years of age: https://www.sciencedirect.com/science/article/pii/S0022347621007368
- Dash, et al. COVID-19 vaccine-induced urticarial vasculitis: https://pubmed.ncbi.nlm.nih.gov/34369046/.
- David, et al. ANCA-associated vasculitis after Oxford AstraZeneca ChAdOx1-S COVID-19 vaccination: a case series of two patients: https://pubmed.ncbi.nlm.nih.gov/34755433/
- De Bruijn, et al. First report of a de novo iTTP episode associated with a COVID-19 mRNA-based anti-COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34105244/
- De Melo Silva, et al. Large hemorrhagic stroke after vaccination against ChAdOx1 nCoV-19: a case report: https://pubmed.ncbi.nlm.nih.gov/34273119/
- De Michele, et al. Malignant cerebral infarction after vaccination with ChAdOx1 nCov-19: a catastrophic variant of vaccine-induced immune-mediated thrombotic thrombocytopenia: https://pubmed.ncbi.nlm.nih.gov/34341358/
- De Vrieze, et al. Pfizer Vaccine Raises Allergy Concerns: https://pubmed.ncbi.nlm.nih.gov/33384356/
- Deb, et al. Acute myocardial injury after COVID-19 vaccination: a case report and review of current evidence from the Vaccine Adverse Event Reporting System database: https://pubmed.ncbi.nlm.nih.gov/34219532/
- Deb, et al. Acute myocardial injury after COVID-19 vaccination: a case report and review of current evidence from the vaccine adverse event reporting system database: https://pubmed.ncbi.nlm.nih.gov/34219532/
- De Dent, et al. Vaccine-induced interstitial lung disease: a rare reaction to COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34510014/.
- Desai, et al. Relationship between pre-existing allergies and anaphylactic reactions following administration of COVID-19 mRNA vaccine: https://pubmed.ncbi.nlm.nih.gov/34215453/
- Dhoot, et al. Thrombocytopenia and splanchnic thrombosis after vaccination with Ad26.COV2.S successfully treated with transjugular intrahepatic intrahepatic portosystemic shunt and thrombectomy: https://onlinelibrary.wiley.com/doi/10.1002/ajh.26258
- Dias, et al. Cerebral venous thrombosis after BNT162b2 mRNA SARS-CoV-2 vaccine: https://www.sciencedirect.com/science/article/abs/pii/S1052305721003098
- Diaz GA, et al. Myocarditis and pericarditis after COVID-19 vaccination: https://jamanetwork.com/journals/jama/fullarticle/2782900
- Diaz GA, Parsons GT, Gering SK, Meier AR, Hutchinson IV, Robicsek A. Myocarditis and Pericarditis After Vaccination for COVID-19. JAMA. 2021 Aug 4.
- Dickey, et al. A series of patients with myocarditis after vaccination against SARS-CoV-2 with mRNA-1279 and BNT162b2: https://www.sciencedirect.com/science/article/pii/S1936878X21004861
- Dicks, et al. Imaging in vascular medicine: leukocytoclastic vasculitis after COVID-19 vaccine booster: https://pubmed.ncbi.nlm.nih.gov/34720009/
- Dimopoulou, D., Spyridis, N., Vartzelis, G., Tsolia, M. N., & Maritsi, D. N. (2021). Safety and tolerability of the COVID-19 mRNA-vaccine in adolescents with juvenile idiopathic arthritis on treatment with TNF-inhibitors. Arthritis Rheumatol. doi:10.1002/art.41977. https://www.ncbi.nlm.nih.gov/pubmed/34492161
- Dimopoulou, D., Vartzelis, G., Dasoula, F., Tsolia, M., & Maritsi, D. (2021). Immunogenicity of the COVID-19 mRNA vaccine in adolescents with juvenile idiopathic arthritis on treatment with TNF inhibitors. Ann Rheum Dis. doi:10.1136/annrheumdis-2021-221607. https://www.ncbi.nlm.nih.gov/pubmed/34844930
- Dionne, et al. Association of myocarditis with COVID-19 messenger RNA vaccine BNT162b2 in a case series of children: https://jamanetwork.com/journals/jamacardiology/fullarticle/2783052
- Douxfils, et al. Hypothesis behind the very rare cases of thrombosis with thrombocytopenia syndrome after SARS-CoV-2 vaccination: https://www.sciencedirect.com/science/article/abs/pii/S0049384821003315
- Dufour, et al. GM1 ganglioside antibody and COVID-19-related Guillain Barre syndrome: case report, systemic review, and implications for vaccine development: https://www.sciencedirect.com/science/article/pii/S2666354621000065
- Dutta, et al. Anti- PF4 antibody negative cerebral venous sinus thrombosis without thrombocytopenia after immunization with COVID-19 vaccine in a non-comorbid elderly Indian male treated with conventional heparin-warfarin based anticoagulation: https://www.sciencedirect.com/science/article/pii/S1871402121002046
- Edler, et al. Deaths associated with the recently launched SARS-CoV-2 vaccination (Comirnaty®): https://pubmed.ncbi.nlm.nih.gov/33895650/
- Ehrlich, et al. Biopsy-proven lymphocytic myocarditis after first COVID-19 mRNA vaccination in a 40-year-old man: case report: https://pubmed.ncbi.nlm.nih.gov/34487236/
- El Sahly, H. M., Baden, L. R., Essink, B., Doblecki-Lewis, S., Martin, J. M., Anderson, E. J., . . . Group, C. S. (2021). Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. N Engl J Med, 385(19), 1774-1785. doi:10.1056/NEJMoa2113017. https://www.ncbi.nlm.nih.gov/pubmed/34551225
- Elrashdy, et al. Autoimmunity Roots of the thrombotic events after COVID-19 vaccination: https://www.sciencedirect.com/science/article/abs/pii/S1568997221002160
- Endo, et al. Central retinal vein occlusion after vaccination with SARS-CoV-2 mRNA: case report: https://pubmed.ncbi.nlm.nih.gov/34571653/.
- Erdem, et al. Acute transverse myelitis following inactivated COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34370410/
- Erler A, et al. A case of leukocytoclastic vasculitis after vaccination with a SARS-CoV2 vaccine: case report: https://pubmed.ncbi.nlm.nih.gov/34196469/.
- Esba, et al. Adverse effects reported after COVID-19 vaccination in a tertiary care hospital, centered on cerebral venous sinus thrombosis (CVST): https://pubmed.ncbi.nlm.nih.gov/34092166/
- Etemadifar, et al. Acute relapse and impaired immunization after COVID-19 vaccination in a patient with multiple sclerosis treated with rituximab: https://pubmed.ncbi.nlm.nih.gov/34015240/
- Facetti, et al. Acute myocarditis in a young adult two days after vaccination with Pfizer: https://pubmed.ncbi.nlm.nih.gov/34709227/
- Faermann, et al. COVID-19 vaccination-induced lymphadenopathy in a specialized breast imaging clinic in Israel: analysis of 163 cases: https://pubmed.ncbi.nlm.nih.gov/34257025/.
- Faissner, et al. COVID-19 mRNA vaccine-induced rhabdomyolysis and fasciitis induced by the: https://pubmed.ncbi.nlm.nih.gov/34435250/
- Falkenhaim-Lopez, et al. Generalized purpura annularis telangiectodes after SARS-CoV-2 mRNA vaccination: https://pubmed.ncbi.nlm.nih.gov/34236717/.
- Fan, et al. Cerebral venous thrombosis following BNT162b2 mRNA vaccination of BNT162b2 against SARS-CoV-2: a black swan event: https://pubmed.ncbi.nlm.nih.gov/34133027/
- Fanni, et al. Severe vaccine-induced thrombotic thrombocytopenia following vaccination with COVID-19: an autopsy case report and review of the literature: https://pubmed.ncbi.nlm.nih.gov/34355379/.
- Fathy, et al Varicella zoster virus and herpes simplex virus reactivation after vaccination with COVID-19: review of 40 cases in an international dermatologic registry: https://pubmed.ncbi.nlm.nih.gov/34487581/
- Favaloro, et al. Laboratory testing for suspicion of COVID-19 vaccine-induced thrombotic (immune) thrombocytopenia: https://pubmed.ncbi.nlm.nih.gov/34138513/
- Fazlollahi, A., Zahmatyar, M., Noori, M., Nejadghaderi, S. A., Sullman, M. J. M., Shekarriz-Foumani, R., . . . Safiri, S. (2021). Cardiac complications following mRNA COVID-19 vaccines: A systematic review of case reports and case series. Rev Med Virol, e2318. doi:10.1002/rmv.2318. https://www.ncbi.nlm.nih.gov/pubmed/34921468
- Fazolo, T., Lima, K., Fontoura, J. C., de Souza, P. O., Hilario, G., Zorzetto, R., . . . Bonorino, C. (2021). Pediatric COVID-19 patients in South Brazil show abundant viral mRNA and strong specific anti-viral responses. Nat Commun, 12(1), 6844. doi:10.1038/s41467-021-27120-y. https://www.ncbi.nlm.nih.gov/pubmed/34824230
- Fearon, et al. Takotsubo syndrome after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34539938/.
- Fernandez-Prada, et al. Acute-onset supraclavicular lymphadenopathy coincident with intramuscular mRNA vaccination against COVID-19 may be related to the injection technique of the vaccine, Spain, January and February 2021: https://pubmed.ncbi.nlm.nih.gov/33706861/
- Fikenzer, S., & Laufs, U. (2021). Correction to: Response to Letter to the editors referring to Fikenzer, S., Uhe, T., Lavall, D., Rudolph, U., Falz, R., Busse, M., Hepp, P., & Laufs, U. (2020). Effects of surgical and FFP2/N95 face masks on cardiopulmonary exercise capacity. Clinical research in cardiology: official journal of the German Cardiac Society, 1-9. Advance online publication. https://doi.org/10.1007/s00392-020-01704-y. Clin Res Cardiol, 110(8), 1352. doi:10.1007/s00392-021-01896-x. https://www.ncbi.nlm.nih.gov/pubmed/34170372
- Finsterer J. Lobar hemorrhage with ventricular rupture shortly after the first dose of an mRNA-based SARS-CoV-2 vaccine: https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8553377/
- Finsterer J. SARS-CoV-2 vaccines are not safe for those with Guillain-Barre syndrome following vaccination: https://www.sciencedirect.com/science/article/pii/S2049080121005343
- Finsterer J. SARS-CoV-2 vaccines can be complicated not only by Guillain-Barré syndrome but also by distal small fiber neuropathy: https://pubmed.ncbi.nlm.nih.gov/34525410/
- Finsterer, et al. Aphasia seven days after the second dose of an mRNA-based SARS-CoV-2 vaccine. Brain MRI revealed an intracerebral hemorrhage (ICBH) in the left temporal lobe in a 52-year-old man. https://www.sciencedirect.com/science/article/pii/S2589238X21000292#f0005
- Finsterer, et al. Parsonage-Turner syndrome associated with SARS-CoV-2 or SARS-CoV-2 vaccination. Comment on: “Neuralgic amyotrophy and COVID-19 infection: 2 cases of accessory spinal nerve palsy” by Coll et al. Articular Spine 2021; 88: 10519: https://pubmed.ncbi.nlm.nih.gov/34139321/.
- Finsterer, et al. Peduncular, symptomatic cavernous bleeding after immune thrombocytopenia-induced SARS-CoV-2 vaccination: https://pubmed.ncbi.nlm.nih.gov/34549178/.
- Flower, et al. Acute ST-segment elevation myocardial infarction secondary to vaccine-induced immune thrombosis with thrombocytopenia (VITT): https://pubmed.ncbi.nlm.nih.gov/34580132/.
- Foltran, et al. Myocarditis and pericarditis in adolescents after the first and second doses of COVID-19 mRNA vaccines: https://pubmed.ncbi.nlm.nih.gov/34849667/
- Forgacs, D., Jang, H., Abreu, R. B., Hanley, H. B., Gattiker, J. L., Jefferson, A. M., & Ross, T. M. (2021). SARS-CoV-2 mRNA Vaccines Elicit Different Responses in Immunologically Naive and Pre-Immune Humans. Front Immunol, 12, 728021. doi:10.3389/fimmu.2021.728021. https://www.ncbi.nlm.nih.gov/pubmed/34646267
- Fousse, et al. Case report: cerebral sinus vein thrombosis in two patients with AstraZeneca SARS-CoV-2 vaccine: https://pubmed.ncbi.nlm.nih.gov/34609603/
- Fowler, et al. Acute-onset central serous retinopathy after immunization with COVID-19 mRNA vaccine. https://www.sciencedirect.com/science/article/pii/S2451993621001456.
- Franchini, et al. Cerebral venous thrombosis and thrombocytopenia after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/33878469/.
- Franchini, et al. Immune thrombosis and thrombocytopenia (VITT) associated with the COVID-19 vaccine: diagnostic and therapeutic recommendations for a new syndrome: https://pubmed.ncbi.nlm.nih.gov/33987882/
- Frank, et al. Prolonged anaphylaxis to Pfizer 2019 coronavirus disease vaccine: a case report and mechanism of action: https://pubmed.ncbi.nlm.nih.gov/33834172/
- Fritzen, et al. Leukocytoclastic vasculitis after exposure to COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34836739/
- Fueryo-Rodriguez, et al. Secondary immune thrombocytopenia putatively attributable to COVID-19 vaccination: https://casereports.bmj.com/content/14/5/e242220.abstract.
- Fujikawa, et al. Neuromyelitis optica in a healthy woman after vaccination against severe acute respiratory syndrome coronavirus 2 mRNA-1273: https://pubmed.ncbi.nlm.nih.gov/34660149/
- Fukushima, et al. A case of sensory ataxic Guillain-Barre syndrome with immunoglobulin G anti-GM1 antibodies after first dose of COVID-19 BNT162b2 mRNA vaccine (Pfizer): https://pubmed.ncbi.nlm.nih.gov/34871447/
- Furer, V., Eviatar, T., Zisman, D., Peleg, H., Paran, D., Levartovsky, D., . . . Elkayam, O. (2021). Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis, 80(10), 1330-1338. doi:10.1136/annrheumdis-2021-220647. https://www.ncbi.nlm.nih.gov/pubmed/34127481
- Furie, et al. Diagnosis and treatment of cerebral venous sinus thrombosis with vaccine-induced immune-immune thrombotic thrombocytopenia: https://pubmed.ncbi.nlm.nih.gov/33914590/
- Gabarin, et al. Venous thromboembolism and mild thrombocytopenia after vaccination with ChAdOx1 nCoV-19: https://pubmed.ncbi.nlm.nih.gov/34384129/
- Gadi, et al. Severe autoimmune hemolytic anemia after receipt of SARS-CoV-2 mRNA vaccine: https://onlinelibrary.wiley.com/doi/10.1111/trf.16672
- Galindo, R., Chow, H., & Rongkavilit, C. (2021). COVID-19 in Children: Clinical Manifestations and Pharmacologic Interventions Including Vaccine Trials. Pediatr Clin North Am, 68(5), 961-976. doi:10.1016/j.pcl.2021.05.004. https://www.ncbi.nlm.nih.gov/pubmed/34538306
- Ganga, et al. Massive cervical lymphadenopathy following vaccination with COVID-19: https://pubmed.ncbi.nlm.nih.gov/34601889/
- Gangat, et al. Cerebral venous thrombosis and myeloproliferative neoplasms: a three-center study of 74 consecutive cases: https://pubmed.ncbi.nlm.nih.gov/34453762/.
- Gangi, et al. Imaging and hematologic findings in thrombosis and thrombocytopenia after vaccination with ChAdOx1 nCoV-19 (AstraZeneca): https://pubmed.ncbi.nlm.nih.gov/34402666/
- Ganzel, et al. Immune thrombocytopenia following Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34155844/
- Gao, et al. Acute transverse myelitis after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34579245/.
- Garcia, et al. Acute myocarditis after administration of BNT162b2 vaccine against COVID-19: https://pubmed.ncbi.nlm.nih.gov/33994339/
- Garcia-Azorin, et al. Delayed headache after COVID-19 vaccination: a warning sign for vaccine-induced cerebral venous thrombosis: https://pubmed.ncbi.nlm.nih.gov/34535076/.
- Gardellini, et al. Severe immune thrombocytopenia following COVID-19 vaccination: report of four cases and review of the literature: https://pubmed.ncbi.nlm.nih.gov/34653943/.
- Garg, et al. Spectrum of neurological complications after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34719776/.
- Gargano, J. W., Wallace, M., Hadler, S. C., Langley, G., Su, J. R., Oster, M. E., . . . Oliver, S. E. (2021). Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices – United States, June 2021. MMWR Morb Mortal Wkly Rep, 70(27), 977-982. doi:10.15585/mmwr.mm7027e2. https://www.ncbi.nlm.nih.gov/pubmed/34237049
- Garreffa, et al. Regional lymphadenopathy after COVID-19 vaccination: review of the literature and considerations for patient management in breast cancer care: https://pubmed.ncbi.nlm.nih.gov/34731748/
- Gatti, M., Raschi, E., Moretti, U., Ardizzoni, A., Poluzzi, E., & Diemberger, I. (2021). Influenza Vaccination and Myo-Pericarditis in Patients Receiving Immune Checkpoint Inhibitors: Investigating the Likelihood of Interaction through the Vaccine Adverse Event Reporting System and VigiBase. Vaccines (Basel), 9(1). doi:10.3390/vaccines9010019. https://www.ncbi.nlm.nih.gov/pubmed/33406694
- Gautam, N., Saluja, P., Fudim, M., Jambhekar, K., Pandey, T., & Al’Aref, S. (2021). A Late Presentation of COVID-19 Vaccine-Induced Myocarditis. Cureus, 13(9), e17890. doi:10.7759/cureus.17890. https://www.ncbi.nlm.nih.gov/pubmed/34660088
- Geeraerts, et al. Cerebral venous thrombosis and vaccine-induced thrombocytopenia.a. Oxford-AstraZeneca COVID-19: a missed opportunity for a rapid return on experience: https://pubmed.ncbi.nlm.nih.gov/34033927/
- Gellad, W. F. (2021). Myocarditis after vaccination against covid-19. BMJ, 375, n3090. doi:10.1136/bmj.n3090. https://www.ncbi.nlm.nih.gov/pubmed/34916217
- Gerber, et al. COVID-19 vaccines induce severe hemolysis in paroxysmal nocturnal hemoglobinuria: https://ashpublications.org/blood/article/137/26/3670/475905/COVID-19-vaccines-induce-severe-hemolysis-in
- Gessler, et al. Neurosurgical considerations regarding decompressive craniectomy for intracerebral hemorrhage after SARS-CoV-2 vaccination in vaccine-induced thrombotic thrombocytopenia-VITT: https://pubmed.ncbi.nlm.nih.gov/34202817/
- Ghielmetti, et al. Acute autoimmune-like hepatitis with atypical antimitochondrial antibody after vaccination with COVID-19 mRNA: a new clinical entity: https://pubmed.ncbi.nlm.nih.gov/34293683/.
- Gillion, et al. Granulomatous vasculitis after AstraZeneca anti-SARS-CoV-2 vaccine: https://pubmed.ncbi.nlm.nih.gov/34237323/.
- Giovane, et al. Bilateral thalamic stroke: a case of COVID-19 (VITT) vaccine-induced immune thrombotic thrombocytopenia or a coincidence due to underlying risk factors: https://pubmed.ncbi.nlm.nih.gov/34820232/.
- Gobel, et al. Headache attributed to COVID-19 (SARS-CoV-2 coronavirus) vaccination with the ChAdOx1 nCoV-19 (AZD1222) vaccine: a multicenter observational cohort study: https://pubmed.ncbi.nlm.nih.gov/34313952/
- Goldman, et al. Rapid progression of angioimmunoblastic T-cell lymphoma after BNT162b2 mRNA booster vaccination: case report: https://www.frontiersin.org/articles/10.3389/fmed.2021.798095/
- Gomez de Terreros Caro, et al. Bell’s palsy after COVID-19 vaccination: case report: https://pubmed.ncbi.nlm.nih.gov/34330676/
- Gonzalez-Romero, et al. Lipschütz ulcers after AstraZeneca COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34366434/.
- Gordon, et al. Immune thrombocytopenia after immunization with Vaxzevria ChadOx1-S vaccine (AstraZeneca), Victoria, Australia: https://pubmed.ncbi.nlm.nih.gov/34756770/
- Graf, et al. Immediate high-dose intravenous immunoglobulins followed by direct treatment with thrombin inhibitors is crucial for survival in vaccine-induced immune thrombotic thrombocytopenia Sars-Covid-19-vector adenoviral VITT with venous thrombosis of the cerebral sinus and portal vein: https://pubmed.ncbi.nlm.nih.gov/34023956/.
- Gras-Champel, et al. Atypical thrombosis associated with the vaccine VaxZevria® (AstraZeneca): data from the French network of regional pharmacovigilance centers: https://pubmed.ncbi.nlm.nih.gov/34083026/.
- Greenhawt, M., Abrams, E. M., Shaker, M., Chu, D. K., Khan, D., Akin, C., . . . Golden, D. B. K. (2021). The Risk of Allergic Reaction to SARS-CoV-2 Vaccines and Recommended Evaluation and Management: A Systematic Review, Meta-Analysis, GRADE Assessment, and International Consensus Approach. J Allergy Clin Immunol Pract, 9(10), 3546-3567. doi:10.1016/j.jaip.2021.06.006. https://www.ncbi.nlm.nih.gov/pubmed/34153517
- Greinacher, et al. Information on ChAdOx1 nCoV-19 vaccine-induced immune-mediated thrombotic thrombocytopenia: https://pubmed.ncbi.nlm.nih.gov/34587242/
- Gresele, et al. Management of cerebral and splanchnic vein thrombosis associated with thrombocytopenia in subjects previously vaccinated with Vaxzevria (AstraZeneca): position statement of the Italian Society for the Study of Hemostasis and Thrombosis (SISET): https://pubmed.ncbi.nlm.nih.gov/33871350/
- Gresele, et al. Interactions of adenoviruess with platelets and coagulation and vaccine-associated autoimmune thrombocytopenia thrombosis syndrome: https://pubmed.ncbi.nlm.nih.gov/34407607/.
- Grossman, et al. IgA vasculitis following COVID-19 vaccination in an adult: https://pubmed.ncbi.nlm.nih.gov/34779011/
- Grupo de trabajo multidisciplinar de FACME: Diagnostic-therapeutic recommendations of the ad-hoc FACME expert working group on the management of cerebral venous thrombosis related to COVID-19 vaccination: https://www.sciencedirect.com/science/article/pii/S0213485321000839
- Guan, et al. A rare case of a middle-age Asian male with cerebral venous thrombosis after COVID-19 AstraZeneca vaccination: https://pubmed.ncbi.nlm.nih.gov/34274191/
- Guerrero, et al. Late cutaneous reactions after administration of COVID-19 mRNA vaccines: https://www.sciencedirect.com/science/article/pii/S2213219821007996
- Gul Rahim, et al. A case of outbreak of macroscopic hematuria and IgA nephropathy after SARS-CoV-2 vaccination: https://pubmed.ncbi.nlm.nih.gov/33932458/
- Gundry SR. Mrna COVID vaccines dramatically increase endothelial inflammatory markers and risk of Acute Coronary Syndrome as measured by PULS cardiac testing: a caution: https://www.ahajournals.org/doi/10.1161/circ.144.suppl_1.10712
- Gunther, et al. Complicated case report of long-term vaccine-induced thrombotic immune thrombocytopenia A: https://pubmed.ncbi.nlm.nih.gov/34835275/.
- Gupta, et al. Covid-19 vaccine-induced thrombosis and thrombocytopenia: a commentary on an important and practical clinical dilemma: https://www.sciencedirect.com/science/article/abs/pii/S0033062021000505
- Gurtler, et al. Cerebral venous thrombosis after COVID-19 vaccination: is the risk of thrombosis increased by intravascular administration of the vaccine: https://pubmed.ncbi.nlm.nih.gov/34286453/.
- Guzman-Perez, et al. Small-vessel vasculitis following Oxford-AstraZeneca vaccination against SARS-CoV-2: https://pubmed.ncbi.nlm.nih.gov/34310763/
- Haaf, P., Kuster, G. M., Mueller, C., Berger, C. T., Monney, P., Burger, P., . . . Tanner, F. C. (2021). The very low risk of myocarditis and pericarditis after mRNA COVID-19 vaccination should not discourage vaccination. Swiss Med Wkly, 151, w30087. doi:10.4414/smw.2021.w30087. https://www.ncbi.nlm.nih.gov/pubmed/34668687
- Haakonsen, et al. Deep venous thrombosis more than two weeks after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/33928773/
- Habib, et al. Acute myocarditis after administration of BNT162b2 vaccine: https://www.sciencedirect.com/science/article/pii/S2214250921001530
- Hafeez, et al. COVID-19 vaccine-associated thrombosis with thrombocytopenia syndrome (TTS): systematic review and post hoc analysis: https://pubmed.ncbi.nlm.nih.gov/34698582/.
- Haimei, et al. Concerns for adverse effects of thrombocytopenia and thrombosis after adenovirus-vectored COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34541935/
- Hajjo, et al. Shedding light on post-vaccination myocarditis and pericarditis in COVID-19 and non-COVID-19 vaccine recipients: https://pubmed.ncbi.nlm.nih.gov/34696294/
- Hakroush, et al. Case report: ANCA-associated vasculitis presenting with rhabdomyolysis and crescentic Pauci-Inmune glomerulonephritis after vaccination with Pfizer-BioNTech COVID-19 mRNA: https://pubmed.ncbi.nlm.nih.gov/34659268/
- Han, et al. Be alert to the risk of adverse cardiovascular events after COVID-19 vaccination: https://www.xiahepublishing.com/m/2472-0712/ERHM-2021-00033
- Hanneman, et al. Evolution of lymphadenopathy on PET/MRI after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/33625301/.
- Hasegawa, et al. Unusual site of deep vein thrombosis after vaccination against coronavirus mRNA-2019 coronavirus disease (COVID-19): https://pubmed.ncbi.nlm.nih.gov/34840204/
- Hashimoto, et al. Elevated rates of anaphylaxis after vaccination with Pfizer BNT162b2 mRNA vaccine against COVID-19 in Japanese healthcare workers; a secondary analysis of initial post-approval safety data: https://pubmed.ncbi.nlm.nih.gov/34128049/
- Hasnie, A. A., Hasnie, U. A., Patel, N., Aziz, M. U., Xie, M., Lloyd, S. G., & Prabhu, S. D. (2021). Perimyocarditis following first dose of the mRNA-1273 SARS-CoV-2 (Moderna) vaccine in a healthy young male: a case report. BMC Cardiovasc Disord, 21(1), 375. doi:10.1186/s12872-021-02183-3. https://www.ncbi.nlm.nih.gov/pubmed/34348657
- Hause, A. M., Gee, J., Baggs, J., Abara, W. E., Marquez, P., Thompson, D., . . . Shay, D. K. (2021). COVID-19 Vaccine Safety in Adolescents Aged 12-17 Years – United States, December 14, 2020-July 16, 2021. MMWR Morb Mortal Wkly Rep, 70(31), 1053-1058. doi:10.15585/mmwr.mm7031e1. https://www.ncbi.nlm.nih.gov/pubmed/34351881
- Hekmat, et al. Potential risk of thrombotic events after COVID-19 vaccination with Oxford-AstraZeneca in women receiving estrogen: https://pubmed.ncbi.nlm.nih.gov/34734086/
- Helmchen, et al. Acute bilateral optic neuritis/chiasm with longitudinal extensive transverse myelitis in long-standing stable multiple sclerosis after vector-based vaccination against SARS-CoV-2: https://pubmed.ncbi.nlm.nih.gov/34131771/
- Helms, et al. Severe and refractory immune thrombocytopenia occurring after SARS-CoV-2 vaccination: https://pubmed.ncbi.nlm.nih.gov/33854395/
- Hendren, et al. Severe myocarditis associated with COVID-19 vaccine: zebra or unicorn?: https://www.internationaljournalofcardiology.com/article/S0167-5273(21)01477-7/fulltext.
- Hermans C, Goldman M. Thrombosis and vaccines: a new challenge in the COVID-19 pandemic. Louvain Med 2021 Apr: 140: 207-215.
- Heymans, S. and L.T. Cooper, Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol, 2021.
- Hidayat, et al. Incidence of acute ischemic stroke after coronavirus vaccination in Indonesia: case series: https://pubmed.ncbi.nlm.nih.gov/34579636/
- Hiller, et al. Lymphadenopathy associated with the COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/33786231/
- Hines, et al. Immune thrombocytopenic purpura and acute liver injury after COVID-19 vaccination: https://casereports.bmj.com/content/14/7/e242678.
- Hinton, et al. Anaphylactoid reaction and coronary thrombosis related to COVID-19 mRNA vaccine: https://pubmed.ncbi.nlm.nih.gov/34863404/.
- Hippisley-Cox, et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and positive SARS-CoV-2 tests: self-controlled case series study: https://pubmed.ncbi.nlm.nih.gov/34446426/
- Ho, J. S., Sia, C. H., Ngiam, J. N., Loh, P. H., Chew, N. W., Kong, W. K., & Poh, K. K. (2021). A review of COVID-19 vaccination and the reported cardiac manifestations. Singapore Med J. doi:10.11622/smedj.2021210. https://www.ncbi.nlm.nih.gov/pubmed/34808708
- Hoeg, et al. Myocarditis associated with SARS-CoV-2 mRNA vaccination in children aged 12 to 17 years: stratified analysis of a national database: https://www.medrxiv.org/content/10.1101/2021.08.30.21262866v1
- Holm, et al. Immune complexes, innate immunity and NETosis in ChAdOx1 vaccine-induced thrombocytopenia: https://pubmed.ncbi.nlm.nih.gov/34405870/.
- Holmes, et al. A case series of skin reactions to COVID-19 vaccine in the Department of Dermatology at Loma Linda University: https://pubmed.ncbi.nlm.nih.gov/34423106/
- Hsiao, et al. Acute transverse myelitis after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34684047/.
- Huh, et al. Predicted and observed incidence of thromboembolic events among Koreans vaccinated with the ChAdOx1 nCoV-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34254476/
- Hundelshausen, et al. Vaccine-induced immune thrombotic immune thrombocytopenia (VITT): targeting pathologic mechanisms with Bruton’s tyrosine kinase inhibitors: https://pubmed.ncbi.nlm.nih.gov/33851389/
- Husby, A., et al., SARS-CoV-2 vaccination and myocarditis or myopericarditis: population-based cohort study. Bmj, 2021. 375: p. e068665.
- Hussain, et al. Deep venous thrombosis after vaccination with Ad26.COV2.S in adult males: https://pubmed.ncbi.nlm.nih.gov/34659839/.
- Huynh, et al. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopenia: https://www.nature.com/articles/s41586-021-03744-4
- Hwang, et al. Comparison of vaccine-induced thrombotic episodes between ChAdOx1 nCoV-19 and Ad26.COV.2.S vaccines: https://www.sciencedirect.com/science/article/abs/pii/S0896841121000895
- Hwang, et al. Predictors of mortality in thrombotic thrombocytopenia after adenoviral COVID-19 vaccination: the FAPIC score: https://pubmed.ncbi.nlm.nih.gov/34545400/
- Hyun, et al. Polyarthralgia and myalgia syndrome after vaccination with ChAdOx1 nCOV-19: https://pubmed.ncbi.nlm.nih.gov/34463066/
- Iba, et al. The roles of platelets in COVID-19-associated coagulopathy and vaccine-induced immune thrombotic immune thrombocytopenia (covid): https://www.sciencedirect.com/science/article/pii/S1050173821000967
- Idogun, et al. Newly diagnosed idiopathic thrombocytopenia after COVID-19 vaccine administration: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8176657/.
- Ifeanyi, et al. Isolated pulmonary embolism after COVID vaccination: 2 case reports and a review of acute pulmonary embolism complications and follow-up: https://pubmed.ncbi.nlm.nih.gov/34804412/
- Iftikhar, et al. Bell’s palsy after 24 hours of mRNA-1273 SARS-CoV-2 mRNA-1273 vaccine: https://pubmed.ncbi.nlm.nih.gov/34336436/
- Igual-Rouilleault, et al. COVID-19 vaccine-induced unilateral axillary adenopathy: follow-up evaluation in the USA: https://pubmed.ncbi.nlm.nih.gov/34655312/.
- Iguchi, et al. Cumulative adverse event report of anaphylaxis following injections of COVID-19 mRNA vaccine (Pfizer-BioNTech) in Japan: the first month report: https://pubmed.ncbi.nlm.nih.gov/34347278/
- Ikenberg, et al. Cerebral venous sinus thrombosis after ChAdOx1 nCov-19 vaccination with a misleading first brain MRI: https://pubmed.ncbi.nlm.nih.gov/34244448/
- In brief: Myocarditis with the Pfizer/BioNTech and Moderna COVID-19 vaccines. (2021). Med Lett Drugs Ther, 63(1629), e9. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/34544112https://www.ncbi.nlm.nih.gov/pubmed/3454412
- Introna, et al. Guillain-Barré syndrome after AstraZeneca COVID-19 vaccination: causal or casual association: https://www.sciencedirect.com/science/article/pii/S0303846721004169
- Ioannou, A. (2021a). Myocarditis should be considered in those with a troponin rise and unobstructed coronary arteries following Pfizer-BioNTech COVID-19 vaccination. QJM. doi:10.1093/qjmed/hcab231. https://www.ncbi.nlm.nih.gov/pubmed/34463755
- Ioannou, A. (2021b). T2 mapping should be utilized in cases of suspected myocarditis to confirm an acute inflammatory process. QJM. doi:10.1093/qjmed/hcab326. https://www.ncbi.nlm.nih.gov/pubmed/34931681
- Iremli, et al. Three cases of subacute thyroiditis after SARS-CoV-2 vaccination: post-vaccination ASIA syndrome: https://pubmed.ncbi.nlm.nih.gov/34043800/.
- Irvine NJ, et al. Petechiae and peeling of fingers after immunization with BTN162b2 messenger RNA (mRNA)-based COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34513435/
- Isaak, A., Feisst, A., & Luetkens, J. A. (2021). Myocarditis Following COVID-19 Vaccination. Radiology, 301(1), E378-E379. doi:10.1148/radiol.2021211766. https://www.ncbi.nlm.nih.gov/pubmed/34342500
- Ismail, et al. A systematic review of cases of CNS demyelination following COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34839149/
- Istampoulouoglou, et al. Myocarditis and pericarditis in association with COVID-19 mRNA vaccination: cases from a regional pharmacovigilance center: https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8587334/
- Iwata, et al. Case of immunoglobulin A vasculitis after vaccination against coronavirus disease 2019: https://pubmed.ncbi.nlm.nih.gov/34535924/
- Izzedine, et al. Nephrotic syndrome and vasculitis after SARS-CoV-2 vaccine: true association or circumstantial: https://pubmed.ncbi.nlm.nih.gov/34245294/.
- Jaafar, R., Boschi, C., Aherfi, S., Bancod, A., Le Bideau, M., Edouard, S., . . . La Scola, B. (2021). High Individual Heterogeneity of Neutralizing Activities against the Original Strain and Nine Different Variants of SARS-CoV-2. Viruses, 13(11). doi:10.3390/v13112177. https://www.ncbi.nlm.nih.gov/pubmed/34834983
- Jabagi, et al. Myocardial infarction, stroke, and pulmonary embolism after BNT162b2 mRNA COVID-19 vaccine in persons aged 75 years or older: https://pubmed.ncbi.nlm.nih.gov/34807248/
- Jain E, et al. Facial diplegia: a rare and atypical variant of Guillain-Barré syndrome and the Ad26.COV2.S vaccine: https://pubmed.ncbi.nlm.nih.gov/34447646/
- Jain, et al. Myocarditis associated with COVID-19 vaccination in adolescents: https://publications.aap.org/pediatrics/article/148/5/e2021053427/181357
- James, et al. Guillain-Barré syndrome after ChAdOx1 nCoV-19 COVID-19 vaccination: a case series: https://pubmed.ncbi.nlm.nih.gov/34548920/
- Jamme, et al. Lethal cerebral venous sinus thrombosis after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/33983464/
- Jasaraj, et al. Immune-mediated thrombocytopenic purpura after Pfizer-BioNTech COVID-19 vaccine in an elderly woman: https://pubmed.ncbi.nlm.nih.gov/34513446/
- Jeon, et al. An outbreak of Still’s disease after COVID-19 vaccination in a 34-year-old patient: https://pubmed.ncbi.nlm.nih.gov/34797392/
- Jeong, et al. Sudden sensorineural hearing loss after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34670143/.
- Jhaveri, R., Adler-Shohet, F. C., Blyth, C. C., Chiotos, K., Gerber, J. S., Green, M., . . . Zaoutis, T. (2021). Weighing the Risks of Perimyocarditis With the Benefits of SARS-CoV-2 mRNA Vaccination in Adolescents. J Pediatric Infect Dis Soc, 10(10), 937-939. doi:10.1093/jpids/piab061. https://www.ncbi.nlm.nih.gov/pubmed/34270752
- Jin, et al. Leukocytoclastic vasculitis after coronavirus disease vaccination 2019: https://pubmed.ncbi.nlm.nih.gov/34713472/803
- John, et al. -19 vaccine in patients with hypercoagulability disorders: a clinical perspective: https://pubmed.ncbi.nlm.nih.gov/34786893/
- Jones, et al. Limb ischemia and pulmonary artery thrombosis after ChAdOx1 nCoV-19 vaccine (Oxford-AstraZeneca): a case of vaccine-induced immune thrombotic thrombocytopenia: https://pubmed.ncbi.nlm.nih.gov/33990339/.
- Joob, et al. Change in blood viscosity after COVID-19 vaccination: estimation for persons with underlying metabolic syndrome: https://pubmed.ncbi.nlm.nih.gov/34868465/
- Julian, et al. Idiopathic thrombocytopenic purpura and the Modern Covid-19 vaccine: https://www.annemergmed.com/article/S0196-0644(21)00122-0/fulltext.
- Kadwalwala, et al. Multimodality imaging and histopathology in a young man presenting with fulminant lymphocytic myocarditis and cardiogenic shock after vaccination with mRNA-1273: https://pubmed.ncbi.nlm.nih.gov/34848416/
- Kafil, et al. COVID-19 mRNA vaccination and development of CMR-confirmed myopericarditis: https://www.medrxiv.org/content/10.1101/2021.09.13.21262182v1.full?s=09
- Kanabar, et al. Guillain-Barre syndrome presenting with facial diplegia following vaccination with COVID-19 in two patients: https://pubmed.ncbi.nlm.nih.gov/34649856/
- Kaneta, et al. Young male with myocarditis after mRNA-1273 coronavirus disease-2019 (COVID-19) mRNA vaccination: https://pubmed.ncbi.nlm.nih.gov/34744118/
- Kaneta, K., Yokoi, K., Jojima, K., Kotooka, N., & Node, K. (2021). Young Male With Myocarditis Following mRNA-1273 Vaccination Against Coronavirus Disease-2019 (COVID-19). Circ J. doi:10.1253/circj.CJ-21-0818. https://www.ncbi.nlm.nih.gov/pubmed/34744118
- Kar, et al. Cutaneous small vessel vasculitis after COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34529877/.
- Kaul, et al. Myocarditis after COVID-19 vaccination: https://www.sciencedirect.com/science/article/pii/S2352906721001603
- Kaulen, et al. Neurological autoimmune diseases after SARS-CoV-2 vaccination: a case series: https://pubmed.ncbi.nlm.nih.gov/34668274/.
- Kawano, et al. Cerebral venous vein/venous sinus thrombosis with thrombocytopenia syndrome after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34373413/
- Kazniak-Pfajfer, et al. Myocarditis associated with COVID-19 vaccination in three adolescent boys: https://pubmed.ncbi.nlm.nih.gov/34851078/.
- Keir, et al. Unique imaging findings of neurologic phantosmia after Pfizer-BioNtech COVID-19 vaccination: a case report: https://pubmed.ncbi.nlm.nih.gov/34096896/
- Kelso JM. IgE-mediated allergy to polyethylene glycol (PEG) as a cause of anaphylaxis to COVID-19 mRNA vaccines: https://pubmed.ncbi.nlm.nih.gov/34318537/
- Kemper, et al. Successful treatment of vaccine-induced immune thrombotic thrombocytopenia in a 26-year-old female patient: https://pubmed.ncbi.nlm.nih.gov/34614491/
- Kenda, et al. Treatment of acute ischemic stroke associated with ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia: https://pubmed.ncbi.nlm.nih.gov/34461442/
- Kerneis, et al. COVID-19 vaccines and myocarditis: https://pubmed.ncbi.nlm.nih.gov/34246566/
- Keshavarz, et al. Lymphadenopathy after COVID-19 vaccination: review of imaging findings: https://pubmed.ncbi.nlm.nih.gov/33985872/
- Khan TA, et al. Bilateral immune-mediated keratolysis after immunization with SARS-CoV-2 recombinant viral vector vaccine: https://pubmed.ncbi.nlm.nih.gov/34483273/.
- Khan, et al. Acute transverse myelitis after SARS-CoV-2 vaccination: case report and review of the literature: https://pubmed.ncbi.nlm.nih.gov/34482455/.
- Kharkar, et al. Asymmetric cutaneous vasculitis after COVID-19 vaccination with unusual preponderance of eosinophils: https://pubmed.ncbi.nlm.nih.gov/34115904/.
- Khayat-Khoei, et al. COVID-19 mRNA vaccine leading to CNS inflammation: a case series: https://link.springer.com/article/10.1007/s00415-021-10780-7
- Kherlopian, et al. A case of toxic epidermal necrolysis after vaccination with ChAdOx1 nCoV-19 (AZD1222): https://pubmed.ncbi.nlm.nih.gov/34751429/.
- Khogali, et al. Unusual presentation of acute pericarditis after vaccination against SARS-COV-2 mRNA-1237 Modern: https://pubmed.ncbi.nlm.nih.gov/34447639/
- Khouri, et al. Adverse event reporting and risk of Bell’s palsy after COVID-19 vaccination: https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00646-0/fulltext.
- Kim B, et al. Supraclavicular lymphadenopathy after COVID-19 vaccination in Korea: serial follow-up by ultrasonography: https://pubmed.ncbi.nlm.nih.gov/34116295/.
- Kim HW, et al. Patients with acute myocarditis after COVID-19 mRNA vaccination: https://jamanetwork.com/journals/jamacardiology/fullarticle/2781602.
- Kim, et al. Cardiac imaging of acute myocarditis after vaccination with COVID-19 mRNA: https://pubmed.ncbi.nlm.nih.gov/34402228/
- Kim, et al. Report of a case of myopericarditis after vaccination with BNT162b2 COVID-19 mRNA in a young Korean male: https://pubmed.ncbi.nlm.nih.gov/34636504/
- Kinariwalla, et al. A case of generalized Sweet’s syndrome with vasculitis triggered by recent vaccination with COVID-19: https://pubmed.ncbi.nlm.nih.gov/34849386/
- King, et al. Myocarditis after SARS-CoV-2 mRNA vaccination, a case series: https://pubmed.ncbi.nlm.nih.gov/34396358/.
- Kircheis R. Coagulopathies after SARS-CoV-2 vaccination may derive from a combined effect of SARS-CoV-2 spike protein and adenovirus vector-activated signaling pathways: https://pubmed.ncbi.nlm.nih.gov/34639132/
- Klein, N. P., Lewis, N., Goddard, K., Fireman, B., Zerbo, O., Hanson, K. E., . . . Weintraub, E. S. (2021). Surveillance for Adverse Events After COVID-19 mRNA Vaccination. JAMA, 326(14), 1390-1399. doi:10.1001/jama.2021.15072. https://www.ncbi.nlm.nih.gov/pubmed/34477808
- Klimek, et al. Allergenic components of the mRNA-1273 vaccine for COVID-19: possible involvement of polyethylene glycol and IgG-mediated complement activation: https://pubmed.ncbi.nlm.nih.gov/33657648/
- Klimek, et al. Severe Allergic Reactions after COVID-19 Vaccination with the Pfizer / BioNTech Vaccine in Great Britain and the USA: Position Statement of the German Allergy Societies: German Medical Association of Allergologists (AeDA), German Society for Allergology and Clinical Immunology (DGAKI) and Society for Pediatric Allergology and Environmental Medicine (GPA): https://pubmed.ncbi.nlm.nih.gov/33643776/
- Klimek, L., Bergmann, K. C., Brehler, R., Pfutzner, W., Zuberbier, T., Hartmann, K., . . . Worm, M. (2021). Practical handling of allergic reactions to COVID-19 vaccines: A position paper from German and Austrian Allergy Societies AeDA, DGAKI, GPA and OGAI. Allergo J Int, 1-17. doi:10.1007/s40629-021-00165-7. https://www.ncbi.nlm.nih.gov/pubmed/33898162
- Know CS, et al. Cerebral venous thrombosis after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34045111/
- Knowlton KU. Insights from a murine model of COVID-19 mRNA vaccine-induced myopericarditis: could accidental intravenous injection of a vaccine induce myopericarditis https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab741/6359059
- Koch, et al. Secondary immune thrombocytopenia (ITP) associated with ChAdOx1 Covid-19 vaccine: case report: https://pubmed.ncbi.nlm.nih.gov/34377889/
- Koh JS, et al. In-hospital observational study of neurological disorders in patients recently vaccinated with COVID-19 mRNA vaccines: https://pubmed.ncbi.nlm.nih.gov/34688190/
- Kohli, U., Desai, L., Chowdhury, D., Harahsheh, A. S., Yonts, A. B., Ansong, A., . . . Ang, J. Y. (2021). mRNA Coronavirus-19 Vaccine-Associated Myopericarditis in Adolescents: A Survey Study. J Pediatr. doi:10.1016/j.jpeds.2021.12.025. https://www.ncbi.nlm.nih.gov/pubmed/34952008
- Koizumi, et al. Myocarditis after COVID-19 mRNA vaccines: https://pubmed.ncbi.nlm.nih.gov/34546329/.
- Kong, et al. Bullous drug eruption after the second dose of COVID-19 mRNA-1273 (Moderna) vaccine: Case report: https://www.sciencedirect.com/science/article/pii/S1876034121001878.
- Konstantinidis I, Tsakiropoulou E, Hähner A, de With K, Poulas K, Hummel T. Olfactory dysfunction after coronavirus disease 2019 (COVID-19) vaccination. Int Forum Allergy Rhinol. 2021 May 28: 10.1002 / alr. 22809.
- Konu, et al. Prevalence of serious adverse events among health care professionals after receiving the first dose of ChAdOx1 nCoV-19 coronavirus vaccine (Covishield) in Togo, March 2021: https://pubmed.ncbi.nlm.nih.gov/34819146/.
- Kostoff, R. N., Calina, D., Kanduc, D., Briggs, M. B., Vlachoyiannopoulos, P., Svistunov, A. A., & Tsatsakis, A. (2021a). Erratum to “Why are we vaccinating children against COVID-19?” [Toxicol. Rep. 8C (2021) 1665-1684 / 1193]. Toxicol Rep, 8, 1981. doi:10.1016/j.toxrep.2021.10.003. https://www.ncbi.nlm.nih.gov/pubmed/34642628
- Kounis, et al. Acute myocardial infarction within 24 hours after COVID-19 vaccination: is Kounis syndrome the culprit: https://pubmed.ncbi.nlm.nih.gov/34702550/
- Kounis, et al. Hypersensitivity Myocarditis and COVID-19 Vaccines: https://pubmed.ncbi.nlm.nih.gov/34856634/.
- Kow CS, et al. Cerebral venous thrombosis following COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34045111/.
- Kragholm, et al. Thrombocytopenia after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34332437/.
- Krajewski, et al. Immune thrombocytopenic purpura associated with COVID-19 mRNA vaccine Pfizer-BioNTech BNT16B2b2: https://pubmed.ncbi.nlm.nih.gov/34077572/
- Krantz, et al. An academic hospital experience assessing the risk of COVID-19 mRNA vaccine using patient’s allergy history: https://www.sciencedirect.com/science/article/pii/S2213219821007972
- Kremsner, P. G., Mann, P., Kroidl, A., Leroux-Roels, I., Schindler, C., Gabor, J. J., . . . Group, C.-N.-S. (2021). Safety and immunogenicity of an mRNA-lipid nanoparticle vaccine candidate against SARS-CoV-2 : A phase 1 randomized clinical trial. Wien Klin Wochenschr, 133(17-18), 931-941. doi:10.1007/s00508-021-01922-y. https://www.ncbi.nlm.nih.gov/pubmed/34378087
- Kreuter, et al. Induction and exacerbation of subacute cutaneous lupus erythematosus erythematosus after mRNA- or adenoviral vector-based SARS-CoV-2 vaccination: https://pubmed.ncbi.nlm.nih.gov/34291477/
- Kripilani, et al. A rare case of Guillain-Barré syndrome after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34671572/
- Krutzke, et al. Process-related impurities in the ChAdOx1 nCov-19 vaccine: https://www.researchsquare.com/article/rs-477964/v1
- Krzywicka, et al. Cerebral venous sinus thrombosis following vaccination against SARS-CoV-2: an analysis of cases reported to the European Medicines Agency: https://pubmed.ncbi.nlm.nih.gov/34293217/
- Kulkarni, et al. Understanding the risk of thrombosis with thrombocytopenia syndrome following Ad26.COV2.S vaccination: https://pubmed.ncbi.nlm.nih.gov/34595694/
- Kustin, T., Harel, N., Finkel, U., Perchik, S., Harari, S., Tahor, M., . . . Stern, A. (2021). Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med, 27(8), 1379-1384. doi:10.1038/s41591-021-01413-7. https://www.ncbi.nlm.nih.gov/pubmed/34127854
- Kuter DJ. Exacerbation of immune thrombocytopenia after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34075578/
- Kuzumi, et al. Genital necrosis with cutaneous thrombosis following vaccination with COVID-19 mRNA: https://pubmed.ncbi.nlm.nih.gov/34839563/
- Kwan, M. Y. W., Chua, G. T., Chow, C. B., Tsao, S. S. L., To, K. K. W., Yuen, K. Y., . . . Ip, P. (2021). mRNA COVID vaccine and myocarditis in adolescents. Hong Kong Med J, 27(5), 326-327. doi:10.12809/hkmj215120. https://www.ncbi.nlm.nih.gov/pubmed/34393110
- Kwon, et al. Autoimmune encephalitis after ChAdOx1-S SARS-CoV-2 vaccination: https://pubmed.ncbi.nlm.nih.gov/34846583/
- Laisuan, et al. Anaphylaxis induced by CoronaVac COVID-19 vaccine: clinical features and results of revaccination: https://pubmed.ncbi.nlm.nih.gov/34675550/.
- Lane, et al. COVID-19 vaccine-related axillary and cervical lymphadenopathy in patients with current or previous breast cancer and other malignancies: cross-sectional imaging findings on MRI, CT and PET-CT: https://pubmed.ncbi.nlm.nih.gov/34719892/
- Larson, et al. Clinical and histopathologic spectrum of delayed adverse skin reactions after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34292611/.
- Lavin, et al. Vaccine-induced immune thrombotic immune thrombocytopenia (VITT): a new clinicopathologic entity with heterogeneous clinical presentations: https://pubmed.ncbi.nlm.nih.gov/34159588/.
- Lazaros, et al. A case series of acute pericarditis after vaccination with COVID-19 in the context of recent reports from Europe and the United States: https://pubmed.ncbi.nlm.nih.gov/34635376/
- Lazaros, et al. The new platform of COVID-19 mRNA vaccine and myocarditis: clues to the possible underlying mechanism: https://pubmed.ncbi.nlm.nih.gov/34312010/
- Leclerc, et al. Minimal change disease with severe acute kidney injury after Oxford-AstraZeneca COVID-19 vaccine: case report: https://pubmed.ncbi.nlm.nih.gov/34242687/.
- Lee, E., Chew, N. W. S., Ng, P., & Yeo, T. J. (2021). Reply to “Letter to the editor: Myocarditis should be considered in those with a troponin rise and unobstructed coronary arteries following PfizerBioNTech COVID-19 vaccination”. QJM. doi:10.1093/qjmed/hcab232. https://www.ncbi.nlm.nih.gov/pubmed/34463770
- Lee, et al. Cerebral venous sinus thrombosis after vaccination: the United Kingdom experience: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)01788-8/fulltext
- Lee, et al. Reports of anaphylaxis after coronavirus disease vaccination 2019, South Korea, February 26-April 30, 2021: https://pubmed.ncbi.nlm.nih.gov/34414880/
- Lee, et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination: https://pubmed.ncbi.nlm.nih.gov/33606296/
- Leerunyakul, et al. Case report: Pityriasis rosea-like rash after vaccination with COVID-19: https://pubmed.ncbi.nlm.nih.gov/34557507/
- Lehman, et al. Unilateral lymphadenopathy after COVID-19 vaccination: a practical management plan for radiologists of all specialties: https://pubmed.ncbi.nlm.nih.gov/33713605/
- Lei Y, Zhang J, Schiavon CR, He M, Chen L, Shen H, Zhang Y, Yin Q, Cho Y, Andrade L, Shadel GS, Hepokoski M, Lei T, Wang H, Zhang J, Yuan JX, Malhotra A , Manor U, Wang S, Yuan ZY, Shyy JY. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circ Res. 2021 Apr 30; 128 (9): 1323-1326.
- Lensen, et al. Hepatitis C virus reactivation after COVID-19 vaccination: a case report: https://pubmed.ncbi.nlm.nih.gov/34512037/
- Leung VS, et al. Rituximab-induced acute lympholysis and pancytopenia following vaccination with COVID-19: https://pubmed.ncbi.nlm.nih.gov/34429981/
- Levin, et al. Myocarditis after COVID-19 vaccination: a case series: https://www.sciencedirect.com/science/article/pii/S0264410X21011725?via%3Dihub.
- Li MH, et al. Myocarditis and pericarditis after COVID-19 vaccination: inequalities in age and vaccine types: https://www.mdpi.com/2075-4426/11/11/1106
- Li, et al. Association of self-reported history of high-risk allergy with allergy symptoms after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34698847/
- Li, et al. Intravenous injection of coronavirus disease 2019 (COVID-19) mRNA vaccine can induce acute myopericarditis in a mouse model: https://t.co/j0IEM8cMXI
- Li, J., Hui, A., Zhang, X., Yang, Y., Tang, R., Ye, H., . . . Zhu, F. (2021). Safety and immunogenicity of the SARS-CoV-2 BNT162b1 mRNA vaccine in younger and older Chinese adults: a randomized, placebo-controlled, double-blind phase 1 study. Nat Med, 27(6), 1062-1070. doi:10.1038/s41591-021-01330-9. https://www.ncbi.nlm.nih.gov/pubmed/33888900
- Liang, et al. Emergence of de novo cutaneous vasculitis after vaccination against coronavirus disease (COVID-19): https://pubmed.ncbi.nlm.nih.gov/34599716/.
- Lim, et al. Anaphylaxis reactions to Pfizer BNT162b2 vaccine: report of 3 cases of anaphylaxis following vaccination with Pfizer BNT162b2: https://pubmed.ncbi.nlm.nih.gov/34579211/
- Lim, et al. Case report: acute fulminant myocarditis and cardiogenic shock after messenger RNA coronavirus vaccination in 2019 requiring extracorporeal cardiopulmonary resuscitation: https://pubmed.ncbi.nlm.nih.gov/34778411/
- Lim, et al. COVID-19 vaccine-related axillary lymphadenopathy in breast cancer patients: case series with literature review: https://pubmed.ncbi.nlm.nih.gov/34836672/.
- Lim, et al. New-onset nephrotic syndrome after Janssen COVID-19 vaccination: case report and literature review: https://pubmed.ncbi.nlm.nih.gov/34342187/.
- Lin CY, et al. Abdominal pain and bilateral adrenal hemorrhage from immune thrombotic thrombocytopenia induced by COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34546343/
- Lin W, et al. Cerebral venous sinus thrombosis, pulmonary embolism, and thrombocytopenia after COVID-19 vaccination in a Taiwanese man: a case report and review of the literature: https://pubmed.ncbi.nlm.nih.gov/34630307/
- Ling, et al. Guillain-Barré syndrome after SARS-CoV-2 vaccination in a patient with previous vaccine-associated Guillain-Barré syndrome: https://pubmed.ncbi.nlm.nih.gov/34810163/
- Lioudaki, et al. Multiple sites of arterial thrombosis in a 35-year-old patient after vaccination with ChAdOx1 (AstraZeneca), which required emergency femoral and carotid surgical thrombectomy: https://pubmed.ncbi.nlm.nih.gov/34644642/
- Lippi, et al. Cerebral venous thrombosis developing after vaccination. COVID-19: VITT, VATT, TTS and more: https://pubmed.ncbi.nlm.nih.gov/34695859/
- Liu, et al. Extensive investigations revealed consistent pathophysiologic alterations after vaccination with COVID-19 vaccines: https://www.nature.com/articles/s41421-021-00329-3
- Long SS. Important insights into myopericarditis after vaccination with Pfizer COVID-19 mRNA in adolescents: https://www.sciencedirect.com/science/article/pii/S0022347621007496
- Long, et al. Thrombosis with thrombocytopenia syndrome associated with COVID-19 vaccines: https://www.sciencedirect.com/science/article/abs/pii/S0735675721004381
- Lorente, et al. Idiopathic external jugular vein thrombophlebitis after coronavirus disease vaccination (COVID-19): https://pubmed.ncbi.nlm.nih.gov/33624509/.
- Luk, et al. Myocarditis and pericarditis after vaccination with COVID-19 mRNA: practical considerations for care providers: https://www.sciencedirect.com/science/article/pii/S0828282X21006243
- Maayan, et al. Acquired thrombotic thrombocytopenic purpura: a rare disease associated with the BNT162b2 vaccine: https://pubmed.ncbi.nlm.nih.gov/34105247/.
- MacIntyre, et al. Thrombosis with thrombocytopenia syndrome (TTS) following AstraZeneca ChAdOx1 nCoV-19 (AZD1222) COVID-19 vaccination: risk-benefit analysis for persons <60 years in Australia: https://pubmed.ncbi.nlm.nih.gov/34272095/
- Madelon, N., Lauper, K., Breville, G., Sabater Royo, I., Goldstein, R., Andrey, D. O., . . . Eberhardt, C. S. (2021). Robust T cell responses in anti-CD20 treated patients following COVID-19 vaccination: a prospective cohort study. Clin Infect Dis. doi:10.1093/cid/ciab954. https://www.ncbi.nlm.nih.gov/pubmed/34791081
- Maeda, et al. Acute myocarditis defined after vaccination with 2019 mRNA of coronavirus disease: https://pubmed.ncbi.nlm.nih.gov/34866122/
- Majid I, et al. Sweet’s syndrome after Oxford-AstraZeneca COVID-19 vaccine (AZD1222) in an elderly woman: https://pubmed.ncbi.nlm.nih.gov/34590397/
- Major, et al. Refractory vaccine-induced immune thrombotic thrombocytopenia (VITT) treated with delayed therapeutic plasma exchange (TPE): https://pubmed.ncbi.nlm.nih.gov/34672380/.
- Maki, et al. Biventricular systolic dysfunction in acute myocarditis after SARS-CoV-2 mRNA-1273 vaccination: https://pubmed.ncbi.nlm.nih.gov/34601566/
- Malamud, et al. Guillain-Barre syndrome after COVID-19 vaccination in an adolescent: https://www.pedneur.com/article/S0887-8994(21)00221-6/fulltext.
- Malayala, et al. A case of idiopathic thrombocytopenic purpura after a booster dose of COVID-19 BNT162b2 vaccine (Pfizer-Biontech): https://pubmed.ncbi.nlm.nih.gov/34820240/
- Malayala, et al. Purpuric rash and thrombocytopenia after mRNA-1273 (Modern) COVID-19 vaccine: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7996471/
- Malik, et al. Pulmonary embolism, transient ischemic attack, and thrombocytopenia after Johnson & Johnson COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34261635/
- Mangat, C., & Milosavljevic, N. (2021). BNT162b2 Vaccination during Pregnancy Protects Both the Mother and Infant: Anti-SARS-CoV-2 S Antibodies Persistently Positive in an Infant at 6 Months of Age. Case Rep Pediatr, 2021, 6901131. doi:10.1155/2021/6901131. https://www.ncbi.nlm.nih.gov/pubmed/34676123
- Maniscalco, et al. Severe relapse of multiple sclerosis after COVID-19 vaccination: a case report: https://pubmed.ncbi.nlm.nih.gov/34447349/
- Mansour, et al. Acute myocarditis after a second dose of COVID-19 mRNA vaccine: report of two cases: https://www.clinicalimaging.org/article/S0899-7071(21)00265-5/fulltext.
- Maramattom, et al. Inflammatory myositis after vaccination with ChAdOx1: https://pubmed.ncbi.nlm.nih.gov/34585145/
- Marchandot, et al. Procoagulant microparticles: a possible link between vaccine-induced immune thrombocytopenia (VITT) and cerebral sinus venous thrombosis: https://pubmed.ncbi.nlm.nih.gov/34129181/.
- Marcucci, et al. Vaccine-induced thrombotic thrombocytopenia: the elusive link between thrombosis and adenovirus-based SARS-CoV-2 vaccines: https://pubmed.ncbi.nlm.nih.gov/34191218/
- Mark, C., Gupta, S., Punnett, A., Upton, J., Orkin, J., Atkinson, A., . . . Alexander, S. (2021). Safety of administration of BNT162b2 mRNA (Pfizer-BioNTech) COVID-19 vaccine in youths and young adults with a history of acute lymphoblastic leukemia and allergy to PEG-asparaginase. Pediatr Blood Cancer, 68(11), e29295. doi:10.1002/pbc.29295. https://www.ncbi.nlm.nih.gov/pubmed/34398511
- Marshall, et al. Acute symptomatic myocarditis in 7 adolescents after Pfizer-BioNTech COVID-19 vaccination. https://pubmed.ncbi.nlm.nih.gov/34088762/.
- Martins-Filho, P. R., Quintans-Junior, L. J., de Souza Araujo, A. A., Sposato, K. B., Souza Tavares, C. S., Gurgel, R. Q., . . . Santos, V. S. (2021). Socio-economic inequalities and COVID-19 incidence and mortality in Brazilian children: a nationwide register-based study. Public Health, 190, 4-6. doi:10.1016/j.puhe.2020.11.005. https://www.ncbi.nlm.nih.gov/pubmed/33316478
- Martin-Villares, et al. Bell’s palsy after a single dose of vaccine mRNA. SARS-CoV-2: case report: https://pubmed.ncbi.nlm.nih.gov/34032902/.
- Mason, et al. Bilateral facial nerve palsy and COVID-19 vaccination: causality or coincidence: https://pubmed.ncbi.nlm.nih.gov/34522557/
- Masset, et al. Pancreas allograft rejection after ChAdOx1 nCoV-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34781027/
- Masuccio, et al. Guillain-Barre syndrome after COVID-19 mRNA-1273 vaccine: case report: https://pubmed.ncbi.nlm.nih.gov/34767184/.
- Matar, et al. Acute hemichorea-hemibalismus after COVID-19 (AZD1222) vaccination: https://pubmed.ncbi.nlm.nih.gov/34581453/
- Matarneh, et al. COVID-19 vaccine causing Guillain-Barre syndrome, an uncommon potential side effect: https://pubmed.ncbi.nlm.nih.gov/34484780/
- Maye, et al. Reactivation of IgA vasculitis after vaccination with COVID-19: https://pubmed.ncbi.nlm.nih.gov/34848431/
- Mayfield, et al. Anaphylaxis after Modern COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34734159/.
- McGonagle, et al. Mechanisms of immunothrombosis in vaccine-induced thrombotic thrombocytopenia (VITT) compared to natural SARS-CoV-2 infection: https://www.sciencedirect.com/science/article/abs/pii/S0896841121000706
- McLean, et al. Myopericarditis in a previously healthy adolescent male after COVID-19 vaccination: Case report: https://pubmed.ncbi.nlm.nih.gov/34133825/
- McMahon, et al. Clinical and pathologic correlates of skin reactions to COVID-19 vaccine, including V-REPP: a registry-based study: https://www.sciencedirect.com/science/article/pii/S0190962221024427
- Mehta, et al. Cerebral venous sinus thrombosis and thrombocytopenia after COVID-19 vaccination: report of two cases in the United Kingdom: https://www.sciencedirect.com/science/article/abs/pii/S088915912100163X
- Mehta, et al. Unilateral axillary adenopathy in the setting of COVID-19 vaccination: follow-up: https://pubmed.ncbi.nlm.nih.gov/34298342/
- Melas N. Portal vein thrombosis occurring after the first dose of SARS-CoV-2 mRNA vaccine in a patient with antiphospholipid syndrome: https://www.sciencedirect.com/science/article/pii/S2666572721000389
- Mendes de Almeida, et al. Intracerebral hemorrhage associated with vaccine-induced thrombotic thrombocytopenia after ChAdOx1 nCOVID-19 vaccination in a pregnant woman: https://pubmed.ncbi.nlm.nih.gov/34261297/
- Mevorach, D., Anis, E., Cedar, N., Bromberg, M., Haas, E. J., Nadir, E., . . . Alroy-Preis, S. (2021). Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel. N Engl J Med, 385(23), 2140-2149. doi:10.1056/NEJMoa2109730. https://www.ncbi.nlm.nih.gov/pubmed/34614328
- Michiels, et al. Humoral response induced by Prime-Boost vaccination with ChAdOx1 nCoV-19 and BNT162b2 mRNA vaccines in a patient with multiple sclerosis treated with teriflunomide: https://pubmed.ncbi.nlm.nih.gov/34696248/
- Min YG, et al. Sensory Guillain-Barré syndrome following ChAdOx1 nCov-19 vaccine: report of two cases and review of the literature: https://pubmed.ncbi.nlm.nih.gov/34416410/.
- Minocha, et al. Recurrence of acute myocarditis temporally associated with receipt of the 2019 coronavirus mRNA disease vaccine (COVID-19) in an adolescent male: https://pubmed.ncbi.nlm.nih.gov/34166671/
- Miqdad, et al. Acute myocarditis after administration of the second dose of BNT162b2 COVID-19 vaccine: https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8599115/
- Miqdadi, et al. Acute eosinophilic pneumonia associated with anti-COVID-19 vaccine AZD1222: https://pubmed.ncbi.nlm.nih.gov/34812326/.
- Mitchell, et al. COVID-19 vaccination and lower cervical lymphadenopathy in two-week neck lump clinic: a follow-up audit: https://pubmed.ncbi.nlm.nih.gov/33947605/.
- Mitchell, et al. Supraclavicular lymphadenopathy after COVID-19 vaccination: an increasing presentation in the two-week wait neck lump clinic: https://pubmed.ncbi.nlm.nih.gov/33685772/
- Mizrahi, B., Lotan, R., Kalkstein, N., Peretz, A., Perez, G., Ben-Tov, A., . . . Patalon, T. (2021). Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nat Commun, 12(1), 6379. doi:10.1038/s41467-021-26672-3. https://www.ncbi.nlm.nih.gov/pubmed/34737312
- Moffitt, K., Cheung, E., Yeung, T., Stamoulis, C., & Malley, R. (2021). Analysis of Staphylococcus aureus Transcriptome in Pediatric Soft Tissue Abscesses and Comparison to Murine Infections. Infect Immun, 89(4). doi:10.1128/IAI.00715-20. https://www.ncbi.nlm.nih.gov/pubmed/33526560
- Moghimi, et al. Allergic reactions and anaphylaxis to LNP-based COVID-19 vaccines: https://pubmed.ncbi.nlm.nih.gov/33571463/
- Mohamed, L., Madsen, A. M. R., Schaltz-Buchholzer, F., Ostenfeld, A., Netea, M. G., Benn, C. S., & Kofoed, P. E. (2021). Reactivation of BCG vaccination scars after vaccination with mRNA-Covid-vaccines: two case reports. BMC Infect Dis, 21(1), 1264. doi:10.1186/s12879-021-06949-0. https://www.ncbi.nlm.nih.gov/pubmed/34930152
- Mohta, et al. Recurrent herpes zoster after COVID-19 vaccination in patients with chronic urticaria on cyclosporine treatment – A report of 3 cases: https://pubmed.ncbi.nlm.nih.gov/34510694/
- Monagle, et al. Vaccine-induced immune thrombosis and thrombocytopenia syndrome after adenovirus-vectored severe acute respiratory syndrome coronavirus 2 vaccination: a new hypothesis on mechanisms and implications for future vaccine development: https://pubmed.ncbi.nlm.nih.gov/34664303/.
- Montgomery, et al. Myocarditis after immunization with COVID-19 mRNA vaccines in members of the U.S. military: https://jamanetwork.com/journals/jamacardiology/fullarticle/2781601%5C
- Morehouse, et al. A rare variant of Guillain-Barré syndrome after vaccination with Ad26.COV2.S: https://pubmed.ncbi.nlm.nih.gov/34703690/.
- Mortazavi, et al. Axillary adenopathy associated with COVID-19 vaccination: imaging findings and follow-up recommendations in 23 women: https://pubmed.ncbi.nlm.nih.gov/33624520/
- Mucke, et al. First description of immune complex vasculitis after COVID-19 vaccination with BNT162b2: case report: https://pubmed.ncbi.nlm.nih.gov/34530771/.
- Muir, et al. Report of the International Cerebral Venous Thrombosis Consortium on cerebral venous thrombosis after SARS-CoV-2 vaccination: https://pubmed.ncbi.nlm.nih.gov/34462996/
- Mungmunpuntipantip, et al. Major artery thrombosis and vaccination against ChAdOx1 nCov-19: https://pubmed.ncbi.nlm.nih.gov/34839830/
- Murakami, et al. Myocarditis following vaccination with COVID-19 messenger RNA: a Japanese case series: https://pubmed.ncbi.nlm.nih.gov/34840235/.
- Mustafa, et al. Comments on thrombosis after vaccination: spike protein leader sequence could be responsible for thrombosis and antibody-mediated thrombocytopenia: https://pubmed.ncbi.nlm.nih.gov/34788138
- Muthukumar, et al. In-depth evaluation of a case of presumed myocarditis after the second dose of COVID-19 mRNA vaccine: https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.121.056038
- Nagasaka, et al. Acute myocarditis associated with COVID-19 vaccination: report of a case: https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8639400/
- Nagrani, et al. Onset / outbreak of psoriasis after Corona virus ChAdOx1 nCoV-19 vaccine (Oxford-AstraZeneca / Covishield): report of two cases: https://pubmed.ncbi.nlm.nih.gov/34350668/
- Naitlho, et al. A rare case of Henoch-Schönlein purpura after a case report of COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34518812/
- Nassar, et al. COVID-19 vaccine-induced myocarditis: a case report with review of the literature: https://www.sciencedirect.com/science/article/pii/S1871402121002253
- Nassar, et al. COVID-19 vaccine-induced rhabdomyolysis: case report with literature review: https://pubmed.ncbi.nlm.nih.gov/34186348/.
- Nastro, et al. Varicella-zoster virus-related small-vessel vasculitis after Pfizer-BioNTech COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34310759/.
- Nasuelli, et al. A case of acute demyelinating polyradiculoneuropathy with bilateral facial palsy following ChAdOx1 nCoV-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34272622/
- Nawwar, et al. Oxford-AstraZeneca COVID-19 vaccination induced lymphadenopathy on [18F] choline PET / CT, not just an FDG finding: https://pubmed.ncbi.nlm.nih.gov/33661328/
- Negrea, et al. Gross hematuria after severe acute respiratory syndrome coronavirus 2 vaccination in 2 patients with IgA nephropathy: https://pubmed.ncbi.nlm.nih.gov/33771584/
- Nevet A. Acute myocarditis associated with anti-COVID-19 vaccination: https://ecevr.org/DOIx.php?id=10.7774/cevr.2021.10.2.196.
- Ng, et al. Ocular adverse events following COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34559576/
- Nishiguchi, et al. Miller Fisher syndrome after 2019 BNT162b2 mRNA coronavirus vaccination: https://pubmed.ncbi.nlm.nih.gov/34789193/.
- Nishizawa, et al. Bell’s palsy after Ad26.COV2.S COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34014316/
- Noseda, et al. Reporting of acute inflammatory neuropathies with COVID-19 vaccines: subgroup disproportionality analysis in VigiBase: https://pubmed.ncbi.nlm.nih.gov/34579259/
- Notghi, et al. Extensive longitudinal transverse myelitis following AstraZeneca COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34507942/.
- Nowak, et al. Neuro-ophthalmic complications with thrombocytopenia and thrombosis induced by ChAdOx1 nCoV-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34726934/
- Ntouros, P. A., Vlachogiannis, N. I., Pappa, M., Nezos, A., Mavragani, C. P., Tektonidou, M. G., . . . Sfikakis, P. P. (2021). Effective DNA damage response after acute but not chronic immune challenge: SARS-CoV-2 vaccine versus Systemic Lupus Erythematosus. Clin Immunol, 229, 108765. doi:10.1016/j.clim.2021.108765. https://www.ncbi.nlm.nih.gov/pubmed/34089859
- Nuovo GJ, Magro C, Shaffer T, Awad H, Suster D, Mikhail S, He B, Michaille JJ, Liechtenstein B, Tili E. Endothelial cell damage is the central part of COVID-19 and a mouse model induced by injection of the S1 subunit of the spike protein. Ann Diagn Pathol. 2021 Apr; 51: 151682.
- Nygaard, U., Holm, M., Bohnstedt, C., Chai, Q., Schmidt, L. S., Hartling, U. B., . . . Stensballe, L. G. (2022). Population-based Incidence of Myopericarditis After COVID-19 Vaccination in Danish Adolescents. Pediatr Infect Dis J, 41(1), e25-e28. doi:10.1097/INF.0000000000003389. https://www.ncbi.nlm.nih.gov/pubmed/34889875
- Obeid, et al. Reactivation of IgA vasculitis after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34250509/
- Oberhardt, V., Luxenburger, H., Kemming, J., Schulien, I., Ciminski, K., Giese, S., . . . Hofmann, M. (2021). Rapid and stable mobilization of CD8(+) T cells by SARS-CoV-2 mRNA vaccine. Nature, 597(7875), 268-273. doi:10.1038/s41586-021-03841-4. https://www.ncbi.nlm.nih.gov/pubmed/34320609
- Obermann, et al. Bell’s palsy after COVID-19 vaccination with high antibody response in CSF: https://pubmed.ncbi.nlm.nih.gov/34322761/.
- Ocal, et al. Portal vein thrombosis associated with ChAdOx1 nCov-19 vaccine: https://www.thelancet.com/journals/langas/article/PIIS2468-1253(21)00197-7/
- Ogata AF, Cheng CA, Desjardins M, Senussi Y, Sherman AC, Powell M, Novack L, Von S, Li X, Baden LR, Walt DR. Circulating SARS-CoV-2 Vaccine Antigen Detected in the Plasma of mRNA-1273 Vaccine Recipients. Clin Infect Dis. 2021 May 20: ciab465.
- Ogbebor, et al. Guillain-Barre syndrome after the first dose of SARS-CoV-2 vaccine: a temporary occurrence, not a causal association: https://pubmed.ncbi.nlm.nih.gov/33968610/
- Okada, et al. Possible triggers of thrombocytopenia and/or hemorrhage by BNT162b2 vaccine, Pfizer-BioNTech: https://pubmed.ncbi.nlm.nih.gov/34660652/.
- Okuda, et al. Propylthiouracil-induced neutrophil anti-cytoplasmic antibody-associated vasculitis after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34451967/
- Onderko, et al. Myocarditis in the setting of a recent COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34712497/.
- Oo, et al. AstraZeneca COVID-19 vaccine and Guillain-Barré syndrome in Tasmania: a causal link: https://pubmed.ncbi.nlm.nih.gov/34560365/
- Ori, et al. Transient cardiac injury in adolescents receiving the BNT162b2 mRNA COVID-19 vaccine: https://journals.lww.com/pidj/Abstract/9000/Transient_Cardiac_Injury_in_Adolesce nts_Receiving.95800.aspx
- Osowicki, et al. Guillain-Barré syndrome in an Australian state using mRNA and adenovirus-vector SARS-CoV-2 vaccines: https://onlinelibrary.wiley.com/doi/10.1002/ana.26218.
- Ostrowski, et al. Inflammation and platelet activation after COVID-19 vaccines: possible mechanisms behind vaccine-induced immune thrombocytopenia and thrombosis: https://pubmed.ncbi.nlm.nih.gov/34887867/.
- Ozdemir, et al. Kounis syndrome type 1 induced by inactivated SARS-COV-2 vaccine: https://pubmed.ncbi.nlm.nih.gov/34148772/
- Ozutemiz, et al. Lymphadenopathy in COVID-19 vaccine recipients: diagnostic dilemma in oncology patients: https://pubmed.ncbi.nlm.nih.gov/33625300/
- Pagenkopf, et al. A case of longitudinally extensive transverse myelitis following Covid-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34182207/
- Palaiodimou, et al. Cerebral venous sinus thrombosis and thrombotic events after vector-based COVID-19 vaccines: systematic review and meta-analysis: https://pubmed.ncbi.nlm.nih.gov/34610990/.
- Pan, et al. Bilateral uveitis after inoculation with COVID-19 vaccine: a case report: https://www.sciencedirect.com/science/article/pii/S1201971221007797
- Panovska-Stavridis, et al. A rare case of thrombosis and thrombocytopenia of the superior ophthalmic vein after ChAdOx1 nCoV-19 vaccination against SARS-CoV-2: https://pubmed.ncbi.nlm.nih.gov/34276917/
- Papasavvas, et al. Reactivation of Vogt-Koyanagi-Harada disease under control for more than 6 years, after anti-SARS-CoV-2 vaccination: https://pubmed.ncbi.nlm.nih.gov/34224024/.
- Pappaterra, et al. Transient oculomotor paralysis after administration of RNA-1273 messenger vaccine for SARS-CoV-2 diplopia after COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34369471/
- Park, et al. Epidemiology and clinical features of myocarditis/pericarditis before the introduction of COVID-19 mRNA vaccine in Korean children: a multicenter study: https://pubmed.ncbi.nlm.nih.gov/34402230/
- Park, et al. Retinal hemorrhage after SARS-CoV-2 vaccination: https://pubmed.ncbi.nlm.nih.gov/34884407/.
- Park, et al. Self-limited myocarditis presenting with chest pain and ST-segment elevation in adolescents after vaccination with the BNT162b2 mRNA vaccine: https://pubmed.ncbi.nlm.nih.gov/34180390/
- Park, et al. Thrombosis and SARS-CoV-2 vaccines: vaccine-induced immune thrombotic thrombocytopenia: https://pubmed.ncbi.nlm.nih.gov/34237213/.
- Patel YR, et al. Cardiovascular magnetic resonance findings in young adult patients with acute myocarditis after COVID-19 mRNA vaccination: a case series: https://pubmed.ncbi.nlm.nih.gov/34496880/
- Patel, et al. Renal vein thrombosis and pulmonary embolism secondary to vaccine-induced thrombotic immune thrombocytopenia (VITT): https://pubmed.ncbi.nlm.nih.gov/34268278/.
- Patone, et al. Neurological complications after the first dose of COVID-19 vaccines and SARS-CoV-2 infection: https://pubmed.ncbi.nlm.nih.gov/34697502/
- Patone, M., Mei, X. W., Handunnetthi, L., Dixon, S., Zaccardi, F., Shankar-Hari, M., . . . Hippisley-Cox, J. (2021). Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. doi:10.1038/s41591-021-01630-0. https://www.ncbi.nlm.nih.gov/pubmed/34907393
- Patone, et al. Risk of myocarditis following sequential COVID-19 vaccinations by age and sex. https://www.medrxiv.org/content/10.1101/2021.12.23.21268276v1.full.pdf
- Patrignani, et al. Acute myocarditis after Comirnaty vaccination in a healthy male with previous SARS-CoV-2 infection: https://pubmed.ncbi.nlm.nih.gov/34367386/
- Paulsen, et al. Immune thrombocytopenic purpura after vaccination with COVID-19 vaccine (ChAdOx1 nCov-19): https://www.sciencedirect.com/science/article/abs/pii/S0006497121013963.
- Pavord, et al. Clinical features of vaccine-induced thrombocytopenia and immune thrombosis: https://pubmed.ncbi.nlm.nih.gov/34379914/.
- Pegat, et al. COVID-19 adenovirus vaccines and Guillain-Barré syndrome with facial palsy: https://onlinelibrary.wiley.com/doi/10.1002/ana.26258.
- Pepe, et al. Myocarditis, pericarditis and cardiomyopathy after COVID-19 vaccination: https://www.sciencedirect.com/science/article/pii/S1443950621011562
- Perez, et al. Myocarditis after 2019 coronavirus disease mRNA vaccine: a case series and determination of incidence rate: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab926/6420408
- Perrotta, A., Biondi-Zoccai, G., Saade, W., Miraldi, F., Morelli, A., Marullo, A. G., . . . Peruzzi, M. (2021). A snapshot global survey on side effects of COVID-19 vaccines among healthcare professionals and armed forces with a focus on headache. Panminerva Med, 63(3), 324-331. doi:10.23736/S0031-0808.21.04435-9. https://www.ncbi.nlm.nih.gov/pubmed/34738774
- Perry, et al. Cerebral venous thrombosis after COVID-19 vaccination in the UK: a multicentre cohort study: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)01608-1/
- Phillips EJ. Allergic reactions after COVID-19 vaccination: putting the risk in perspective: https://pubmed.ncbi.nlm.nih.gov/34463751/
- Pinana, J. L., Lopez-Corral, L., Martino, R., Montoro, J., Vazquez, L., Perez, A., . . . Cell Therapy, G. (2022). SARS-CoV-2-reactive antibody detection after SARS-CoV-2 vaccination in hematopoietic stem cell transplant recipients: Prospective survey from the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group. Am J Hematol, 97(1), 30-42. doi:10.1002/ajh.26385. https://www.ncbi.nlm.nih.gov/pubmed/34695229
- Pitlick, et al. Biphasic anaphylaxis after first dose of 2019 messenger RNA coronavirus disease vaccine with positive polysorbate 80 skin test result: https://pubmed.ncbi.nlm.nih.gov/34343674/
- Placke, et l. Coronavirus disease vaccine 2019 mimics lymph node metastases in patients undergoing skin cancer follow-up: a single-center study: https://pubmed.ncbi.nlm.nih.gov/34280870/
- Plasse, et al. Acute kidney injury with macroscopic hematuria and IgA nephropathy after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34352309/
- Plaza, et al. Unilateral axillary lymphadenopathy related to COVID-19 vaccine: pattern on screening breast MRI allowing benign evaluation: https://pubmed.ncbi.nlm.nih.gov/34325221/
- Pomara, et al. COVID-19 vaccine and death: causality algorithm according to the WHO eligibility diagnosis: https://pubmed.ncbi.nlm.nih.gov/34073536/
- Pomara, et al. Post-mortem findings in vaccine-induced thrombotic thrombocytopenia (covid-19): https://haematologica.org/article/view/haematol.2021.279075
- Porres-Aguilar, et al. COVID-19 vaccine-induced immune-immune thrombotic thrombocytopenia: an emerging cause of splanchnic vein thrombosis: https://www.sciencedirect.com/science/article/pii/S1665268121000557
- Portuguesa, et al. Collection of complement-mediated and autoimmune-mediated hematologic conditions after SARS-CoV-2 vaccination: https://ashpublications.org/bloodadvances/article/5/13/2794/476324/Autoimmune-and-complement-mediated-hematologic
- Pottegard, et al. Arterial events, venous thromboembolism, thrombocytopenia and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population-based cohort study: https://pubmed.ncbi.nlm.nih.gov/33952445/
- Prasad, et al. A novel case of bifacial diplegia variant of Guillain-Barré syndrome after vaccination with Janssen COVID-19: https://pubmed.ncbi.nlm.nih.gov/34449715/
- Premkumar, et al. New portal vein thrombosis in cirrhosis: is thrombophilia exacerbated by vaccine or COVID-19: https://www.jcehepatology.com/article/S0973-6883(21)00545-4/fulltext.
- Purkayasha, et al. Rare case of COVID-19 vaccine-associated intracranial hemorrhage with venous sinus thrombosis: https://pubmed.ncbi.nlm.nih.gov/34556531/.
- Qasim, et al. Relapse of immune thrombocytopenia after covid-19 vaccination in young male patient: https://pubmed.ncbi.nlm.nih.gov/34804803/.
- Raj, et al. Subclinical axillary lymphadenopathy associated with COVID-19 vaccination on screening mammography: https://pubmed.ncbi.nlm.nih.gov/34906409/
- Ramdeny, et al. Management of a patient with a rare congenital limb malformation syndrome after SARS-CoV-2 vaccine-induced thrombosis and thrombocytopenia (VITT): https://pubmed.ncbi.nlm.nih.gov/34097311/
- Ramessur, et al. Cutaneous thrombosis associated with cutaneous necrosis following Oxford-AstraZeneca COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34189756/
- Ramirez-Garcia, et al. Pericarditis after administration of BNT162b2 mRNA COVID-19 mRNA vaccine: https://www.sciencedirect.com/science/article/pii/S1885585721002218
- Ramos, et al. Acral hemorrhage after administration of the second dose of SARS-CoV-2 vaccine. A post-vaccination reaction: https://pubmed.ncbi.nlm.nih.gov/34092400/742.
- Rao, et al. A case of Guillain-Barré syndrome after Pfizer COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34567447/
- Rela, et al. Autoimmune hepatitis after COVID vaccine: https://pubmed.ncbi.nlm.nih.gov/34225251/
- Repajic, et al. Bell’s palsy after the second dose of the Pfizer COVID-19 vaccine in a patient with a history of recurrent Bell’s palsy: https://www.sciencedirect.com/science/article/pii/S266635462100020X
- Rerknimitr, et al. Cutaneous adverse reactions of 35,229 doses of COVID-19 Sinovac and AstraZeneca vaccine COVID-19: a prospective cohort study in health care workers: https://pubmed.ncbi.nlm.nih.gov/34661934/
- Restivo, et al. Polyethylene glycole allergy of the SARS CoV2 vaccine recipient: case report of a young adult recipient and management of future exposure to SARS-CoV2: https://pubmed.ncbi.nlm.nih.gov/33919151/
- Revon-Riviere, G., Ninove, L., Min, V., Rome, A., Coze, C., Verschuur, A., . . . Andre, N. (2021). The BNT162b2 mRNA COVID-19 vaccine in adolescents and young adults with cancer: A monocentric experience. Eur J Cancer, 154, 30-34. doi:10.1016/j.ejca.2021.06.002. https://www.ncbi.nlm.nih.gov/pubmed/34233234
- Reyes-Capo, et al. Acute abducens nerve palsy after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34044114/.
- Rhea EM, Logsdon AF, Hansen KM et al. The S1 Protein of SARS-CoV-2 Crosses the Blood-Brain Barrier in Mice. Nature Neuroscience 2021; 24: 368-378.
- Ring, et al. Risk of severe allergic reactions to COVID-19 vaccines among patients with allergic skin disease: practical recommendations. An ETFAD position statement with external experts: https://pubmed.ncbi.nlm.nih.gov/33752263/
- Robichaud, et al. Systemic capillary extravasation syndrome after vaccination with ChAdOx1 nCOV-19 (Oxford-AstraZeneca): https://pubmed.ncbi.nlm.nih.gov/34362727/
- Robinson, et al. Incidence of axillary adenopathy on breast imaging after vaccination with COVID-19: https://pubmed.ncbi.nlm.nih.gov/34292295/.
- Rocco A, Sgamato C, Compare D, Nardone G. Autoimmune hepatitis following SARS-CoV-2 vaccine: May not be a casualty. J Hepatol. 2021 Jun 9: S0168-8278 (21) 00412-8.
- Rodriguez, et al. Lethal vaccine-induced immune thrombotic immune thrombocytopenia (VITT) following announcement 26.COV2.S: first documented case outside the U.S.: https://pubmed.ncbi.nlm.nih.gov/34626338/
- Rodriguez-Pardo, et al. Thrombosis and thrombocytopenia syndrome causing isolated symptomatic carotid occlusion after COVID-19 Ad26.COV2.S vaccine (Janssen): https://pubmed.ncbi.nlm.nih.gov/34670287/
- Roman, et al. Acute transverse myelitis (ATM): clinical review of 43 patients with COVID-19-associated ATM and 3 serious adverse events of post-vaccination ATM with ChAdOx1 nCoV-19 vaccine (AZD1222): https://pubmed.ncbi.nlm.nih.gov/33981305/
- Rose, et al. A report of myocarditis adverse events in the U.S. Vaccine Adverse Event Reporting System. (VAERS) in association with COVID-19 injectable biologics: https://pubmed.ncbi.nlm.nih.gov/34601006/
- Rosner, et al. Myocarditis temporally associated with COVID-19 vaccination: https://www.ahajournals.org/doi/pdf/10.1161/CIRCULATIONAHA.121.055891.
- Rossetti, et al. Guillain-Barré syndrome presenting as facial diplegia after vaccination with COVID-19: a case report: https://www.sciencedirect.com/science/article/pii/S0736467921006442
- Rossi, et al. Recurrence of alopecia areata after covid-19 vaccination: a report of three cases in Italy: https://pubmed.ncbi.nlm.nih.gov/34741583/
- Rzymski, et al. Thrombotic thrombocytopenia after vaccination with COVID-19: in search of the underlying mechanism: https://pubmed.ncbi.nlm.nih.gov/34071883/
- Salah, et al. COVID-19 vaccine and myocarditis: https://pubmed.ncbi.nlm.nih.gov/34399967/
- Saleh, et al. Case study of thrombosis and thrombocytopenia syndrome after administration of the AstraZeneca COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34781321/
- Salih, et al. Vaccine-induced thrombocytopenia with severe headache: https://pubmed.ncbi.nlm.nih.gov/34525282/
- Sanchez Tijmes, F., Thavendiranathan, P., Udell, J. A., Seidman, M. A., & Hanneman, K. (2021). Cardiac MRI Assessment of Nonischemic Myocardial Inflammation: State of the Art Review and Update on Myocarditis Associated with COVID-19 Vaccination. Radiol Cardiothorac Imaging, 3(6), e210252. doi:10.1148/ryct.210252. https://www.ncbi.nlm.nih.gov/pubmed/34934954
- Sanchez van Kammen, et al. Characteristics and outcomes of patients with cerebral venous sinus thrombosis in thrombotic immune thrombocytopenia induced by SARS-CoV-2 vaccine: https://jamanetwork.com/journals/jamaneurology/fullarticle/2784622
- Sandhu, et al. Leukocytoclastic vasculitis as a cutaneous manifestation of ChAdOx1 corona virus vaccine nCoV-19 (recombinant): https://pubmed.ncbi.nlm.nih.gov/34546608/
- Sangli, et al. Thrombosis with thrombocytopenia after messenger RNA vaccine -1273: https://pubmed.ncbi.nlm.nih.gov/34181446/
- Santin AD. VITT (vaccine-induced immune thrombotic thrombocytopenia) after vaccination with ChAdOx1 nCoV-19: https://pubmed.ncbi.nlm.nih.gov/34731555/
- Saraceno, et al. Vogt-Koyanagi-Harada syndrome after COVID-19 and ChAdOx1 nCoV-19 (AZD1222) vaccination: https://pubmed.ncbi.nlm.nih.gov/34462013/.
- Sato, et al. Facial nerve palsy after administration of COVID-19 mRNA vaccines: analysis of self-report database: https://pubmed.ncbi.nlm.nih.gov/34492394/
- Scavone, et al. Platelet activation and modulation in thrombosis with thrombocytopenia syndrome associated with the ChAdO × 1 nCov-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34474550/
- Schauer, et al. Myopericarditis after Pfizer messenger ribonucleic acid coronavirus disease vaccine in adolescents: https://pubmed.ncbi.nlm.nih.gov/34228985/
- Schierz, et al. Vasculitis and bursitis in [ 18 F] FDG-PET/CT after COVID-19 mRNA vaccine: post hoc ergo propter hoc; https://pubmed.ncbi.nlm.nih.gov/34495381/.
- Schmitt, et al. Acute myocarditis after COVID-19 vaccination: a case report: https://www.sciencedirect.com/science/article/pii/S0248866321007098
- Schneider, et al. Post-mortem investigation of deaths after vaccination with COVID-19 vaccines: https://pubmed.ncbi.nlm.nih.gov/34591186/
- Schramm, R., Costard-Jackle, A., Rivinius, R., Fischer, B., Muller, B., Boeken, U., . . . Gummert, J. (2021). Poor humoral and T-cell response to two-dose SARS-CoV-2 messenger RNA vaccine BNT162b2 in cardiothoracic transplant recipients. Clin Res Cardiol, 110(8), 1142-1149. doi:10.1007/s00392-021-01880-5. https://www.ncbi.nlm.nih.gov/pubmed/34241676
- Schultz, et al. Thrombotic thrombocytopenia after vaccination with ChAdOx1 nCov-19: https://www.nejm.org/doi/full/10.1056/NEJMoa2104840?query=recirc_curatedRelated_article
- Schulz JB, et al. Cerebral venous thrombosis associated with COVID-19 vaccine in Germany: https://pubmed.ncbi.nlm.nih.gov/34288044/.
- Scott, et al. Gastroparesis after Pfizer-BioNTech COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34187985/.
- See, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after vaccination with Ad26.COV2.S (against covid-19), March 2 to April 21, 2020: https://pubmed.ncbi.nlm.nih.gov/33929487/
- Sekar, et al. ANCA glomerulonephritis following Modern COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34081948/
- Sellaturay, et al. Polyethylene glycol (PEG) is a cause of anaphylaxis to Pfizer / BioNTech mRNA COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/33825239/
- Seneff S and Nigh G. Worse Than the Disease? Reviewing Some Possible Unintended Consequences of the mRNA Vaccines. International Journal of Vaccine Theory, Practice, and Research 2 (1), May 10, 2021, Page | 38
- Sepahvand, et al. Longitudinally extensive cervical myelitis after vaccination with inactivated virus based COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34849183/
- Sessa, et al. Thromboembolic events in younger females exposed to Pfizer-BioNTech or Moderna COVID-19 vaccines: https://pubmed.ncbi.nlm.nih.gov/34264151/
- Sessa, F., Salerno, M., Esposito, M., Di Nunno, N., Zamboni, P., & Pomara, C. (2021). Autopsy Findings and Causality Relationship between Death and COVID-19 Vaccination: A Systematic Review. J Clin Med, 10(24). doi:10.3390/jcm10245876. https://www.ncbi.nlm.nih.gov/pubmed/34945172
- Shahrigharahkoshan, et al. Induction of cutaneous leukocytoclastic vasculitis after ChAdOx1 nCoV-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34853744/.
- Shakoor, et al. ANCA-associated vasculitis after Pfizer-BioNTech COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34280507/.
- Shao, et al. Guillain-Barré syndrome associated with COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34648420/.
- Sharff, et al. Risk of Myopericarditis following COVID-19 mRNA vaccination in a Large Integrated Health System: A Comparison of Completeness and Timeliness of Two Methods https://www.medrxiv.org/content/10.1101/2021.12.21.21268209v
- Sharif, N., Alzahrani, K. J., Ahmed, S. N., & Dey, S. K. (2021). Efficacy, Immunogenicity and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis. Front Immunol, 12, 714170. doi:10.3389/fimmu.2021.714170. https://www.ncbi.nlm.nih.gov/pubmed/34707602
- Sharifan-Dorche, et al. Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis after covid-19 vaccination; a systematic review: https://pubmed.ncbi.nlm.nih.gov/34365148/.
- Shaw, et al. Possible Association Between COVID-19 Vaccine and Myocarditis: Clinical and CMR Findings: https://pubmed.ncbi.nlm.nih.gov/34246586/
- Shay, D. K., Gee, J., Su, J. R., Myers, T. R., Marquez, P., Liu, R., . . . Shimabukuro, T. T. (2021). Safety Monitoring of the Janssen (Johnson & Johnson) COVID-19 Vaccine – United States, March-April 2021. MMWR Morb Mortal Wkly Rep, 70(18), 680-684. doi:10.15585/mmwr.mm7018e2. https://www.ncbi.nlm.nih.gov/pubmed/33956784
- Shay, et al. Myocarditis occurring after immunization with COVID-19 mRNA-based COVID-19 vaccines: https://jamanetwork.com/journals/jamacardiology/fullarticle/2781600
- Shazley, O., & Alshazley, M. (2021). A COVID-Positive 52-Year-Old Man Presented With Venous Thromboembolism and Disseminated Intravascular Coagulation Following Johnson & Johnson Vaccination: A Case-Study. Cureus, 13(7), e16383. doi:10.7759/cureus.16383. https://www.ncbi.nlm.nih.gov/pubmed/34408937
- Shemer, et al. COVID-19 vaccination association and facial nerve palsy: A case-control study: https://pubmed.ncbi.nlm.nih.gov/34165512/
- Shemer, et al. Peripheral facial nerve palsy after vaccination with BNT162b2 (COVID-19): https://pubmed.ncbi.nlm.nih.gov/33734623/
- Shen, et al. Acute pericarditis and cardiac tamponade after vaccination with Covid-19: https://pubmed.ncbi.nlm.nih.gov/34749492/
- Shimabukuro T. COVID-19 Vaccine Safety Updates Vaccines and Related Biological Products Advisory Committee (VRBPAC). https://www.fda.gov/media/150054/download
- Shimabukuro T. Allergic reactions, including anaphylaxis, after receiving the first dose of Pfizer-BioNTech COVID-19 vaccine – United States, December 14 to 23, 2020: https://pubmed.ncbi.nlm.nih.gov/33641264/
- Shimabukuro, et al. Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons: https://www.nejm.org/doi/full/10.1056/NEJMoa2104983.
- Shin, et al. Lymphadenopathy associated with COVID-19 vaccination on FDG PET/CT: distinguishing features in adenovirus-vectored vaccine: https://pubmed.ncbi.nlm.nih.gov/34115709/.
- Shiyovich, A., Witberg, G., Aviv, Y., Eisen, A., Orvin, K., Wiessman, M., . . . Hamdan, A. (2021). Myocarditis following COVID-19 vaccination: magnetic resonance imaging study. Eur Heart J Cardiovasc Imaging. doi:10.1093/ehjci/jeab230. https://www.ncbi.nlm.nih.gov/pubmed/34739045
- Sim, et al. Coronavirus disease vaccine 2019 (COVID-19) in systemic lupus erythematosus and neutrophil anti-cytoplasmic antibody-associated vasculitis: https://pubmed.ncbi.nlm.nih.gov/33928459/
- Simone, et al. Acute myocarditis after vaccination with COVID-19 mRNA in adults aged 18 years or older: https://pubmed.ncbi.nlm.nih.gov/34605853/
- Simpson, et al. First dose of ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic, and hemorrhagic events in Scotland: https://www.nature.com/articles/s41591-021-01408-4
- Simsek, et al. Massive cerebral venous thrombosis and venous basin infarction as late complications of COVID-19: a case report: https://pubmed.ncbi.nlm.nih.gov/34373991/
- Singer, M. E., Taub, I. B., & Kaelber, D. C. (2021). Risk of Myocarditis from COVID-19 Infection in People Under Age 20: A Population-Based Analysis. medRxiv. doi:10.1101/2021.07.23.21260998. https://www.ncbi.nlm.nih.gov/pubmed/34341797
- Singh, et al. COVID-19 mRNA vaccination and myocarditis: https://pubmed.ncbi.nlm.nih.gov/34268277/
- Singh, et al. COVID-19 vaccine-induced axillary and pectoral lymphadenopathy in PET: https://www.sciencedirect.com/science/article/pii/S1930043321002612
- Sirufo, et al. Henoch-Schönlein purpura following the first dose of COVID-19 viral vector vaccine: case report: https://pubmed.ncbi.nlm.nih.gov/34696186/.
- Smadja, et al. Vaccination against COVID-19: information on the occurrence of arterial and venous thrombosis using data from VigiBase: https://pubmed.ncbi.nlm.nih.gov/33863748/
- Smith, C., Odd, D., Harwood, R., Ward, J., Linney, M., Clark, M., . . . Fraser, L. K. (2021). Deaths in children and young people in England after SARS-CoV-2 infection during the first pandemic year. Nat Med. doi:10.1038/s41591-021-01578-1. https://www.ncbi.nlm.nih.gov/pubmed/34764489
- Snapiri, et al. Transient cardiac injury in adolescents receiving the BNT162b2 mRNA COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34077949/
- Soeiro, et al. Type I interferons as a potential mechanism linking COVID-19 mRNA vaccines with Bell’s palsy: https://pubmed.ncbi.nlm.nih.gov/33858693/
- Somiya, et al. Sex differences in the incidence of anaphylaxis to LNP-mRNA vaccines COVID-19: https://pubmed.ncbi.nlm.nih.gov/34020815/
- Sookaromdee, et al. COVID-19 vaccine, immune thrombotic thrombocytopenia, jaundice, hyperviscosity: concern in cases with underlying hepatic problems: https://pubmed.ncbi.nlm.nih.gov/34509271/.
- Sorensen, et al. A case of multiple thrombocytopenia and thrombosis following vaccination with ChAdOx1 nCoV-19 against SARS-CoV-2: https://pubmed.ncbi.nlm.nih.gov/34137813/
- Sorvoll, et al. An observational study to identify the prevalence of thrombocytopenia and anti-PF4 / polyanion antibodies in Norwegian health care workers after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/33909350/
- Spinner, J. A., Julien, C. L., Olayinka, L., Dreyer, W. J., Bocchini, C. E., Munoz, F. M., & Devaraj, S. (2021). SARS-CoV-2 anti-spike antibodies after vaccination in pediatric heart transplantation: A first report. J Heart Lung Transplant. doi:10.1016/j.healun.2021.11.001. https://www.ncbi.nlm.nih.gov/pubmed/34911654
- Sriwastava, et al. Spectrum of neuroimaging findings in post-CoVID-19 vaccination: a case series and review of the literature: https://pubmed.ncbi.nlm.nih.gov/34842783/
- Stare ova, J., Bluemke, D. A., Bradham, W. S., Grist, T. M., Schiebler, M. L., & Reeder, S. B. (2021). Myocarditis Associated with mRNA COVID-19 Vaccination. Radiology, 301(2), E409-E411. doi:10.1148/radiol.2021211430. https://www.ncbi.nlm.nih.gov/pubmed/34282971
- Starekova, et al. Myocarditis associated with COVID-19 mRNA vaccination: https://pubs.rsna.org/doi/10.1148/radiol.2021211430
- Stassi, et al. A look at the role of postmortem immunohistochemistry in understanding the inflammatory pathophysiology of COVID-19 disease and vaccine-related thrombotic adverse events: a narrative review: https://pubmed.ncbi.nlm.nih.gov/34769454/
- Strobel, et al. Portal vein thrombosis due to vaccine-induced immune thrombotic immune thrombocytopenia (VITT) after Covid vaccination with ChAdOx1 nCoV-19: https://pubmed.ncbi.nlm.nih.gov/34598301/
- Su et al. Case report: vaccine-induced immune thrombotic thrombocytopenia in a patient with pancreatic cancer after vaccination with messenger RNA-1273: https://pubmed.ncbi.nlm.nih.gov/34790684/
- Sulemankhil, et al. Temporal association between COVID-19 vaccine Ad26.COV2.S and acute myocarditis: case report and review of the literature: https://www.sciencedirect.com/science/article/pii/S1553838921005789
- Sumi, et al. Squamous cell carcinoma of the lung with hemoptysis following vaccination with tozinameran (BNT162b2, Pfizer-BioNTech): https://pubmed.ncbi.nlm.nih.gov/34612003/
- Sung, et al. Acute myocardial infarction within 24 hours after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34364657/.
- Suzuki YJ, Gychka SG. SARS-CoV-2 Spike Protein Elicits Cell Signaling in Human Host Cells: Implications for Possible Consequences of COVID-19 Vaccines. Vaccines (Basel). 2021 Jan 11; 9 (1): 36.
- Syed, et al. Central venous sinus thrombosis with subarachnoid hemorrhage after COVID-19 mRNA vaccination: are these reports merely coincidental: https://pubmed.ncbi.nlm.nih.gov/34478433/
- Tahir, et al. Transverse myelitis induced by SARS-CoV-2 vaccination: https://pubmed.ncbi.nlm.nih.gov/34458035/
- Tailor, et al. Case report: acute myocarditis after second dose of mRNA-1273 SARS-CoV-2 mRNA vaccine: https://academic.oup.com/ehjcr/article/5/8/ytab319/6339567
- Tajstra, et al. Acute thrombosis of the coronary tree after vaccination against COVID-19: https://www.sciencedirect.com/science/article/abs/pii/S1936879821003988
- Takase, et al. Chest pain with abnormal electrocardiogram redevelopment after injection of COVID-19 vaccine manufactured by Moderna: https://pubmed.ncbi.nlm.nih.gov/34866106/
- Takeda, M., Ishio, N., Shoji, T., Mori, N., Matsumoto, M., & Shikama, N. (2021). Eosinophilic Myocarditis Following Coronavirus Disease 2019 (COVID-19) Vaccination. Circ J. doi:10.1253/circj.CJ-21-0935. https://www.ncbi.nlm.nih.gov/pubmed/34955479
- Takeyama, et al. Intracerebral hemorrhage due to vasculitis following COVID-19 vaccination: case report: https://pubmed.ncbi.nlm.nih.gov/34783899/
- Tan WY, et al. Extensive longitudinal transverse myelitis after ChAdOx1 nCOV-19 vaccine: case report: https://pubmed.ncbi.nlm.nih.gov/34641797/.
- Tan, et al. COVID-19 post-vaccination lymphadenopathy: report of fine-needle aspiration biopsy cytologic findings: https://pubmed.ncbi.nlm.nih.gov/34432391/
- Tano, et al. Perimyocarditis in adolescents after Pfizer-BioNTech COVID-19 vaccine: https://academic.oup.com/jpids/advance-article/doi/10.1093/jpids/piab060/6329543
- Tarawneh, et al. Immune thrombocytopenia in a 22-year-old post Covid-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/33476455/
- Taylor P, et al. Vaccine-induced thrombosis and thrombocytopenia with bilateral adrenal haemorrhage: https://pubmed.ncbi.nlm.nih.gov/34235757/.
- Team, C. C.-R., Food, & Drug, A. (2021). Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine – United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep, 70(2), 46-51. doi:10.15585/mmwr.mm7002e1. https://www.ncbi.nlm.nih.gov/pubmed/33444297
- Thachil J. COVID-19 vaccine-induced immune thrombosis with thrombocytopenia thrombosis (VITT) and shades of gray in thrombus formation: https://pubmed.ncbi.nlm.nih.gov/34624910/
- Thambiah, et al. Clinical and biological features of cerebral venous sinus thrombosis after vaccination with ChAdOx1 nCov-19; https://jnnp.bmj.com/content/early/2021/09/29/jnnp-2021-327340.
- Theuriet, et al. Guillain-Barre syndrome after the first injection of ChAdOx1 nCoV-19 vaccine: first report: https://pubmed.ncbi.nlm.nih.gov/34217513/.
- Thompson, M. G., Burgess, J. L., Naleway, A. L., Tyner, H., Yoon, S. K., Meece, J., . . . Gaglani, M. (2021). Prevention and Attenuation of Covid-19 with the BNT162b2 and mRNA-1273 Vaccines. N Engl J Med, 385(4), 320-329. doi:10.1056/NEJMoa2107058. https://www.ncbi.nlm.nih.gov/pubmed/34192428
- Tiede, et al. Prothrombotic immune thrombocytopenia after COVID-19 vaccination: https://www.sciencedirect.com/science/article/pii/S0006497121009411
- Tinoco, et al. Perimyocarditis after vaccination with COVID-19: https://pubmed.ncbi.nlm.nih.gov/34866957/
- Tobaiqy, et al. Thrombotic adverse events reported for Moderna, Pfizer, and Oxford-AstraZeneca COVID-19 vaccines: comparison of occurrence and clinical outcomes in the EudraVigilance database: https://pubmed.ncbi.nlm.nih.gov/34835256/
- Toida, et al. Takotsubo cardiomyopathy after coronavirus 2019 vaccination in patient on maintenance hemodialysis: https://pubmed.ncbi.nlm.nih.gov/34731486/.
- Tresckow, et al. hyperplasia after Covid-19 mRNA-based vaccination with Covid-19: https://pubmed.ncbi.nlm.nih.gov/34462647/
- Trimboli, et al. Guillain-Barre syndrome after BNT162b2 COVID-19 vaccine: https://link.springer.com/article/10.1007%2Fs10072-021-05523-5.
- Trogstad, et al. Association between ChAdOx1 nCoV-19 vaccination and bleeding episodes: large population-based cohort study: https://pubmed.ncbi.nlm.nih.gov/34479760/.
- Truong, et al. Clinical suspicion of myocarditis temporally related to COVID-19 vaccination in adolescents and young adults: https://www.ahajournals.org/doi/abs/10.1161/CIRCULATIONAHA.121.056583?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
- Tsilingiris, et al. Vaccine-induced thrombotic thrombocytopenia: the shady chapter of a success story: https://www.sciencedirect.com/science/article/pii/S2589936821000256
- Tun, et al. Immune-mediated hepatitis with the Moderna vaccine is no longer a coincidence but confirmed: https://www.sciencedirect.com/science/article/pii/S0168827821020936
- Turi, et al. A case of vaccine-induced immune-immune thrombotic thrombocytopenia with massive arteriovenous thrombosis: https://pubmed.ncbi.nlm.nih.gov/34059191/
- Turk VE. Anaphylaxis Associated with COVID-19 mRNA Vaccines: Approach to Allergy Research: https://pubmed.ncbi.nlm.nih.gov/33932618/
- Turner, et al. COVID-19 vaccine-associated anaphylaxis: a statement from the Anaphylaxis Committee of the World Allergy Organization: https://www.sciencedirect.com/science/article/pii/S1939455121000119.
- Tutar, et al. A variant of Guillain-Barré syndrome after SARS-CoV-2 vaccination: AMSAN: https://pubmed.ncbi.nlm.nih.gov/34370408/.
- Tutor, A., Unis, G., Ruiz, B., Bolaji, O. A., & Bob-Manuel, T. (2021). Spectrum of Suspected Cardiomyopathy Due to COVID-19: A Case Series. Curr Probl Cardiol, 46(10), 100926. doi:10.1016/j.cpcardiol.2021.100926. https://www.ncbi.nlm.nih.gov/pubmed/34311983
- Tuyls, et al. Allergic reactions to COVID-19 vaccines: statement of the Belgian Society of Allergy and Clinical Immunology (BelSACI): https://www.tandfonline.com/doi/abs/10.1080/17843286.2021.1909447
- Uaprasert, et al. ChAdOx1 nCoV-19 vaccine-associated thrombocytopenia: three cases of immune thrombocytopenia after 107,720 doses of ChAdOx1 vaccination in Thailand: https://pubmed.ncbi.nlm.nih.gov/34483267/.
- Ujueta, et al. Lymphohistocytic myocarditis after vaccination with COVID-19 Ad26.COV2.S viral vector: https://www.sciencedirect.com/science/article/pii/S2352906721001573
- Umei, T. C., Kishino, Y., Shiraishi, Y., Inohara, T., Yuasa, S., & Fukuda, K. (2021). Recurrence of myopericarditis following mRNA COVID-19 vaccination in a male adolescent. CJC Open. doi:10.1016/j.cjco.2021.12.002. https://www.ncbi.nlm.nih.gov/pubmed/34904134
- Underdown, et al. Thrombocytopenia in an adolescent with sickle cell anemia after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34331506/
- Ungari, et al. Cutaneous lymphocytic vasculitis after administration of the second dose of AZD1222 (Oxford-AstraZeneca) Severe acute respiratory syndrome Coronavirus 2 vaccine: chance or causality: https://pubmed.ncbi.nlm.nih.gov/34726187/.
- Uvais NA. Depression after ChAdOx1-S / nCoV-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34608345/.
- Vaira, et al. Secondary thrombocytopenia after SARS-CoV-2 vaccination: case report of haemorrhage and hematoma after minor oral surgery: https://pubmed.ncbi.nlm.nih.gov/34314875/.
- Vaishya, et al. Occurrence of COVID-19 variants among recipients of ChAdOx1 nCoV-19 vaccine (recombinant): https://pubmed.ncbi.nlm.nih.gov/34528522/
- Van Dijk, et al. Relapse of immune thrombocytopenia after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34591991/
- Varas, et al. Venous sinus thrombosis after vaccination with ChAdOx1 nCov-19: https://pubmed.ncbi.nlm.nih.gov/34420802/
- Varona, et al. Primary adrenal insufficiency associated with Oxford-AstraZeneca ChAdOx1 nCoV-19 (VITT) vaccine-induced immune thrombotic thrombocytopenia: https://pubmed.ncbi.nlm.nih.gov/34256983/
- Vassallo, et al. Cutaneous lymphocytic vasculitis after administration of COVID-19 mRNA vaccine: https://pubmed.ncbi.nlm.nih.gov/34327795
- Vayne, et al. PF4 immunoassays in vaccine-induced thrombotic thrombocytopenia: https://www.nejm.org/doi/full/10.1056/NEJMc2106383
- Vegezzi, et al. Acute myelitis and ChAdOx1 nCoV-19 vaccine: coincidental or causal association: https://www.sciencedirect.com/science/article/pii/S0165572821002137
- Vera-Lastra, et al. Two cases of Graves’ disease after SARS-CoV-2 vaccination: an autoimmune / inflammatory syndrome induced by adjuvants: https://pubmed.ncbi.nlm.nih.gov/33858208/
- Verma, et al. Myocarditis following immunization with Covid-19 mRNA: https://www.nejm.org/doi/full/10.1056/NEJMc2109975
- Vidula, et al. Myocarditis and other cardiovascular complications of mRNA-based COVID-19 vaccines: https://pubmed.ncbi.nlm.nih.gov/34277198/
- Villa, et al. A case of ANCA-associated vasculitis after AZD1222 (Oxford-AstraZeneca) SARS-CoV-2 vaccination: victim or causality?: https://pubmed.ncbi.nlm.nih.gov/34416184/.
- Violi, et al. Thrombosis in pre- and post-vaccination phase of COVID-19; https://pubmed.ncbi.nlm.nih.gov/34650382/
- Visclosky, et al. Myocarditis Following mRNA COVID-19 Vaccine: https://journals.lww.com/pec-online/Abstract/2021/11000/Myocarditis_Following_ mRNA_COVID_19_Vaccine.9.aspx.
- Visentini, et al. Outbreaks of mixed cryoglobulinemia vasculitis after vaccination against SARS-CoV-2: https://pubmed.ncbi.nlm.nih.gov/34819272/
- Viskin, et al. Myocarditis associated with COVID-19 vaccination: echocardiographic, cardiac CT, and MRI findings: https://pubmed.ncbi.nlm.nih.gov/34428917/.
- Vogrig, et al. Acute disseminated encephalomyelitis following vaccination against SARS-CoV-2: https://pubmed.ncbi.nlm.nih.gov/34325334/
- Volk, et al. Acute facial paralysis as a possible complication of SARS-CoV-2 vaccination: https://pubmed.ncbi.nlm.nih.gov/33975372/.
- Vollmann, et al. Acute perimyocarditis after the first dose of COVID-19 mRNA vaccine: https://pubmed.ncbi.nlm.nih.gov/34515024/
- Vuille-Lessard, et al. Autoimmune hepatitis triggered by vaccination against SARS-CoV-2: https://pubmed.ncbi.nlm.nih.gov/34332438/
- Waggiallah HA. Thrombosis formation after COVID-19 vaccination immunologic aspects: review article: https://pubmed.ncbi.nlm.nih.gov/34629931/
- Waheed, et al. Neurological complications of COVID-19: Guillain-Barre syndrome after Pfizer COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/33758714/
- Walter, et al. Carotid artery immune thrombosis induced by adenovirus-vectored COVID-19 vaccine: case report: https://pubmed.ncbi.nlm.nih.gov/34312301/.
- Walter, et al. Cerebral venous sinus thrombosis after COVID-19 vaccination : Neurological and radiological management: https://pubmed.ncbi.nlm.nih.gov/34327553/.
- Wan, et al. Bell’s palsy after vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and a nested case-control study: https://pubmed.ncbi.nlm.nih.gov/34411532/
- Wang RL, et al. Acute cerebral venous thrombosis and pulmonary artery embolism associated with the COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34247246/.
- Wantavornprasert, et al. Widespread fixed bullous drug eruption after vaccination with ChAdOx1 nCoV-19: https://pubmed.ncbi.nlm.nih.gov/34482558/
- Waraich, et al. Hematuria, a generalized petechial rash and headaches after Oxford AstraZeneca ChAdOx1 nCoV-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34620638/
- Warkentin, et al. Spontaneous HIT syndrome: knee replacement, infection, and parallels with vaccine-induced immune thrombotic thrombocytopenia: https://pubmed.ncbi.nlm.nih.gov/34144250/
- Warren, C. M., Snow, T. T., Lee, A. S., Shah, M. M., Heider, A., Blomkalns, A., . . . Nadeau, K. C. (2021). Assessment of Allergic and Anaphylactic Reactions to mRNA COVID-19 Vaccines With Confirmatory Testing in a US Regional Health System. JAMA Netw Open, 4(9), e2125524. doi:10.1001/jamanetworkopen.2021.25524. https://www.ncbi.nlm.nih.gov/pubmed/34533570
- Washington, et al. Adenopathy after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/33625299/.
- Watad, et al. Immune-mediated disease outbreaks or recent-onset disease in 27 subjects after mRNA/DNA vaccination against SARS-CoV-2: https://pubmed.ncbi.nlm.nih.gov/33946748/
- Watchmaker, et al. Brain death in a vaccinated patient with COVID-19 infection: https://pubmed.ncbi.nlm.nih.gov/34656887/
- Watkins, et al. Myocarditis after vaccination with BNT162b2 in a healthy male: https://www.sciencedirect.com/science/article/pii/S0735675721005362
- Watts, et al. Case series of vaccine-induced thrombotic thrombocytopenia in a London teaching hospital: https://pubmed.ncbi.nlm.nih.gov/34694650/
- Weeks, et al. Evolution of bilateral hypermetabolic axillary hypermetabolic lymphadenopathy on FDG PET/CT after 2-dose COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34735411/
- Weitzman, E. R., Sherman, A. C., & Levy, O. (2021). SARS-CoV-2 mRNA Vaccine Attitudes as Expressed in U.S. FDA Public Commentary: Need for a Public-Private Partnership in a Learning Immunization System. Front Public Health, 9, 695807. doi:10.3389/fpubh.2021.695807. https://www.ncbi.nlm.nih.gov/pubmed/34336774
- Welsh, et al. Thrombocytopenia, including immune thrombocytopenia after receiving COVID-19 mRNA vaccines reported to the Vaccine Adverse Event Reporting System (VAERS): https://pubmed.ncbi.nlm.nih.gov/34006408/
- Wiedmann, et al. Vaccine-induced immune thrombotic thrombocytopenia causing a severe form of cerebral venous thrombosis with high mortality rate: a case series: https://pubmed.ncbi.nlm.nih.gov/34393988/.
- Wiest, et al. A case of acute pulmonary embolism after immunization with SARS-CoV-2 mRNA: https://pubmed.ncbi.nlm.nih.gov/34452028/
- Williams, et al. Acute myocarditis after SARS-CoV-2 mRNA-1273 mRNA vaccination: https://pubmed.ncbi.nlm.nih.gov/34308326/.
- Wilting, et al. Intracerebral hemorrhage and thrombocytopenia after AstraZeneca COVID-19 vaccine: clinical and diagnostic challenges of vaccine-induced thrombotic thrombocytopenia: https://pubmed.ncbi.nlm.nih.gov/34646685/
- Witberg, G., Barda, N., Hoss, S., Richter, I., Wiessman, M., Aviv, Y., . . . Kornowski, R. (2021). Myocarditis after Covid-19 Vaccination in a Large Health Care Organization. N Engl J Med, 385(23), 2132-2139. doi:10.1056/NEJMoa2110737. https://www.ncbi.nlm.nih.gov/pubmed/34614329
- Wolf, et al. Thrombocytopenia and intracranial venous sinus thrombosis after exposure to the “AstraZeneca COVID-19 vaccine”: https://pubmed.ncbi.nlm.nih.gov/33918932/
- Wolthers, et al. Intracerebral hemorrhage twelve days after vaccination with ChAdOx1 nCoV-19: https://pubmed.ncbi.nlm.nih.gov/34477089/
- Wong, et al. Clinical Guidance for Young People with Myocarditis and Pericarditis after Vaccination with COVID-19 mRNA: https://www.cps.ca/en/documents/position/clinical-guidance-for-youth-with-myocarditis-and-pericarditis
- Woo, et al. Association of receipt association of Ad26.COV2.S COVID-19 vaccine with presumed Guillain-Barre syndrome, February-July 2021: https://jamanetwork.com/journals/jama/fullarticle/2785009
- Wyrozumska K. COV2-S vaccination may reveal hereditary thrombophilia: massive cerebral venous sinus thrombosis in a young man with normal platelet count: https://pubmed.ncbi.nlm.nih.gov/34632750/
- Xu, et al. COVID-19 mRNA vaccination-induced lymphadenopathy mimics lymphoma progression on FDG PET / CT: https://pubmed.ncbi.nlm.nih.gov/33591026/
- Yagi, et al. Cerebral venous sinus thrombosis after mRNA-based COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34783932/.
- Yahyavi-Firouz-Abadi, et al. Cerebral venous sinus thrombosis associated with vaccine-induced thrombotic thrombocytopenia: https://pubmed.ncbi.nlm.nih.gov/34333995/
- Yamaguchi, et al. Cerebral venous sinus thrombosis after vaccination with COVID-19 mRNA of BNT162b2: https://pubmed.ncbi.nlm.nih.gov/34796065/.
- Yocum, et al. Thrombotic thrombocytopenic purpura after vaccination with Ad26.COV2-S: https://pubmed.ncbi.nlm.nih.gov/33980419/
- Yu, et al. Bell’s palsy after inactivated vaccination with COVID-19 in a patient with a history of recurrent Bell’s palsy: case report: https://pubmed.ncbi.nlm.nih.gov/34621891/
- Zakaria, et al. Cerebral venous sinus thrombosis 2 weeks after first dose of SARS-CoV-2 mRNA vaccine: https://pubmed.ncbi.nlm.nih.gov/34101024/
- Zakaria, et al. Cerebral venous sinus thrombosis 2 weeks after the first dose of SARS-CoV-2 mRNA vaccine: https://pubmed.ncbi.nlm.nih.gov/34101024/.
- Zhang S, Liu Y, Wang X, Yang L, Li H, Wang Y, Liu M, Zhao X, Xie Y, Yang Y, Zhang S, Fan Z, Dong J, Yuan Z, Ding Z, Zhang Y, Hu L SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol. 2020 Sep 4; 13 (1): 120.
- Zheng, et al. Acute retinal necrosis due to varicella zoster virus reactivation after vaccination with BNT162b2 COVID-19 mRNA: https://pubmed.ncbi.nlm.nih.gov/34851795/.
- Zuhorn, et al. Post-vaccinal encephalitis after ChAdOx1 nCov-19: https://pubmed.ncbi.nlm.nih.gov/34324214/
- Karikó, K., Muramatsu, H., Welsh, F. A., Ludwig, J., Kato, H., Akira, S., & Weissman, D. (2008). Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Molecular therapy : the journal of the American Society of Gene Therapy, 16(11), 1833–1840. https://doi.org/10.1038/mt.2008.200
- Mulligan, M.J., Lyke, K.E., Kitchin, N. et al. Publisher Correction: Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 590, E26 (2021). https://doi.org/10.1038/s41586-020-03098-3
- https://childrenshealthdefense.org/defender/covid-vaccine-spike-protein-travels-from-injection-site-organ-damage/ (Byram Bridle)
- https://www.nytimes.com/2021/01/12/health/covid-vaccine-death.html
- Kanduc, D., Shoenfeld, Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine. Immunol Res 68, 310–313 (2020). https://doi.org/10.1007/s12026-020-09152-6
- Shoenfeld Y. Corona (COVID-19) time musings: Our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmunity Reviews 19 (2020) 102538. https://doi.org/10.1016/j.autrev.2020.102538
- Ehrenfeld M, et al., Autoimmunity Reviews, https://doi.org/10.1016/j.autrev.2020.102597
- Rose J, Crawford M. Estimating the number of COVID vaccine deaths in America [Internet]. Available from: https://downloads.regulations.gov/CDC-2021-0089-0024/attachment_1.pdf
- Classen, J. Bart. “COVID-19 RNA Based Vaccines and the Risk of Prion Disease.” Microbiol Infect Dis 5.1 (2021): 1-3.
- Classen, J. Bart. “Review of COVID-19 Vaccines and the Risk of Chronic Adverse Events Including Neurological Degeneration.” Journal of Medical-Clinical Research and Reviews 5.4 (2021): 1-7.
- Sucharit Bhakdi: Massive self-to-self attack of immune system. Note: start at the 2:46 minute mark: https://rairfoundation.com/dr-sucharit-bhakdi-vaccine-benefit-zero-fears-massive-self-to-self-attack-of-immune-system-video/
- Dr. Arne Burkhardt: 2nd Pathology Conference—Are Deaths and Adverse Health Effects after Vaccination against COVID-19 related in a Pathologically detectable way? https://odysee.com/@en:a5/Pathology-Conference-2-en:b Note: The most important portion of Dr. Burkhardt’s presentation extends from the 16:30 minute mark to the 52:36-minute mark.
- Pantazatos SP, Seligmann H. COVID vaccination and age-stratified all-cause mortality risk. Preprint. Researchgate-2021.
- VAERSanalysis: VAERS summary for COVID-19 vaccines through 1/7/22. https://vaersanalysis.info/2022/01/14/vaers-summary-for-covid-19-vaccines-through-01-07-2022/
- Rose J. VAERS Update: Presentation for the Canadian COVID Care Alliance. XXXXXXXX
- CDC Report, September 2, 2021: Selected Adverse Events Reported after COVID-19 Vaccination. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html
- National Vaccine Information Center: https://www.medalerts.org/vaersdb/findfield.php?TABLE=ON&GROUP1=AGE&EVENTS=ON&VAX=COVID19&DIED=Yes
- Hause, et al. COVID-19 Vaccine Safety in Adolescents Aged 12–17 Years — United States, December 14, 2020–July 16, 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7031e1.htm?s_cid=mm7031e1_e&ACSTrackingID=USCDC_921-DM62612&ACSTrackingLabel=MMWR%20Early%20Release%20-%20Vol.%2070%2C%20July%2030%2C%202021&deliveryName=USCDC_921-DM62612#contribAff
- MEDCHECK: Mortality risk of vaccination is 7 times higher than that of COVID-19 in [persons in their] 20s. Bulletin of the Japanese Institute of Pharmacovigilance https://www.npojip.org/english/MedCheck/Med%20Check%20Tip-20-2021-08&12.pdf
- Sorg, e t al. Risk of Hospitalization, severe disease, and mortality due to COVID-19 and PIMS-TS in children with SARS-CoV-2 infection in Germany. https://www.medrxiv.org/content/10.1101/2021.11.30.21267048v1
- Ludvigsson, et al. Open schools, COVID-19, and child and teacher morbidity in Sweden. https://www.nejm.org/doi/pdf/10.1056/NEJMc2026670?articleTools=true
- Smith, et al. Deaths in Children and Young People in England following SARS-CoV-2 infection during the first pandemic year: a national study using linked mandatory child death reporting data. medRxiv preprint doi: https://doi.org/10.1101/2021.07.07.21259779;
- CDC: Provisional COVID-19 Deaths: Focus on ages 0-18. https://data.cdc.gov/NCHS/Provisional-COVID-19-Deaths-Focus-on-Ages-0-18-Yea/nr4s-juj3
- Tartof, et al. Lancet 2021, 398, 1407-1416.
- Kostoff RN, Calina D, Kanduc D, et al. Why are we vaccinating children against COVID-19? [published correction appears in Toxicol Rep. 2021;8:1981]. Toxicol Rep. 2021;8:1665-1684. doi:10.1016/j.toxrep.2021.08.010
- Polack, F. P. et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine 383, 2603–2615 (2020).
- Krammer F. A correlate of protection for SARS-CoV-2 vaccines is urgently needed. Nat Med 2021 277 [Internet] 2021 [cited 2021 Aug 9];27(7):1147–8. Available from: https://www.nature.com/articles/s41591-021-01432-4
- Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol [Internet] 2020;5(12):1598–607. Available from: https://doi.org/10.1038/s41564-020-00813-8
- Ruopp MD, Strymish J, Dryjowicz-Burek J, Creedon K, Gupta K. Durability of SARS-CoV-2 IgG Antibody Among Residents in a Long-Term Care Community. J Am Med Dir Assoc [Internet] 2021;22(3):510–1. Available from: https://pubmed.ncbi.nlm.nih.gov/33515497
- Seligmann H. Expert evaluation on adverse effects of the Pfizer-COVID-19 vaccination. https://in-this-together.com/ukc/Selligmann-Yativ-Paper.pdf
- SARS-CoV-2 variants of concern and variants under investigation in England. Technical briefing 22, Sep 3, 2021. Public Health England. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1014926/Technical_Briefing_22_21_09_02.pdf
- Brown CM, et al. Outbreak of SARS-CoV-2 Infections, Including COVID-19 Vaccine Breakthrough Infections, Associated with Large Public Gatherings — Barnstable County, Massachusetts, July 2021. US Department of Health and Human Services/Centers for Disease Control and Prevention MMWR / August 6, 2021 / Vol. 70 / No. 31.
- Chau NVV, et al. Transmission of SARS-CoV-2 Delta Variant Among Vaccinated Healthcare Workers, Vietnam. Lancet Preprint. Available at SSRN: https://ssrn.com/abstract=3897733 or http://dx.doi.org/10.2139/ssrn.3897733
- Hetemaki, et al. An outbreak caused by the SARS-CoV-2 Delta variant (B.1.617.2) in a secondary care hospital in Finland, May 2021. https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2021.26.30.2100636
- Salvatore, et al. Transmission potential of vaccinated and unvaccinated persons infected with the SARS-CoV-2 Delta variant in a federal prison, July—August 2021. https://www.medrxiv.org/content/10.1101/2021.11.12.21265796v1
- Acharya, et al. No Significant Difference in Viral Load Between Vaccinated and Unvaccinated, Asymptomatic and Symptomatic Groups When Infected with SARS-CoV-2 Delta Variant. https://www.medrxiv.org/content/10.1101/2021.09.28.21264262v2
- Riemersma, et al. Vaccinated and unvaccinated individuals have similar viral loads in communities with high prevalence of SARS-CoV-2 delta variant. medRxiv preprint doi: https://doi.org/10.1101/2021.07.31.21261387
- Riemersma, et al. Shedding of infectious SARS-CoV-2 despite vaccination when delta variant is prevalent. medRxiv preprint doi: https://doi.org/10.1101/2021.07.31.21261387
- Chia, et al. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine-breakthrough infections: a multi-center cohort study. https://www.medrxiv.org/content/10.1101/2021.07.28.21261295v1
- Chemaitelly, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. https://www.medrxiv.org/content/10.1101/2021.08.25.21262584v1.full.pdf
- Nordstrom, et al. The impact of SARS-CoV-2 vaccination on Alpha & Delta variant transmission. https://www.medrxiv.org/content/10.1101/2021.09.28.21264260v1
- Canaday, et al. Significant reduction in humoral immunity among healthcare workers and nursing home residents 6 months after COVID-19 BNT162b2 mRNA vaccination. https://www.medrxiv.org/content/10.1101/2021.08.15.21262067v3
- Eyre, et al. The impact of SARS-CoV-2 vaccination on Alpha & Delta variant transmission. https://www.medrxiv.org/content/10.1101/2021.09.28.21264260v1
- Yahi, et al. Infection-enhancing anti-SARS-CoV-2 antibodies recognize both the original Wuhan/D614G strain and Delta variants. A potential risk for mass vaccination? https://www.journalofinfection.com/article/S0163-4453(21)00392-3/fulltext
- Public Health Scotland COVID-19 & Winter Statistical Report January 19, 2022: https://publichealthscotland.scot/media/11223/22-01-19-covid19-winter_publication_report.pdf
- Ontario COVID-19 data: https://covid-19.ontario.ca/data
- Australia COVID-19 data: NSW Health COVID-19 Critical Intelligence Unit: COVID-19 Monitor, 13 January 2022https://aci.health.nsw.gov.au/__data/assets/pdf_file/0008/698804/20220113-COVID-19-Monitor.pdf
- Wang, et al. https://www.medrxiv.org/content/10.1101/2022.01.12.22269179v1.full.pdf
- Lee, S.H. A Routine Sanger Sequencing Target Specific Mutation Assay for SARS-CoV-2 Variants of Concern and Interest. Viruses 2021, 13, 2386. https://doi.org/10.3390/ v13122386
- Reid AH, et al. 1918 Influenza pandemic caused by highly conserved viruses with two receptor-binding variants. Emerging Infectious Diseases. 2003, Vol 9, #10, p. 1249. https://pubmed.ncbi.nlm.nih.gov/16494711/
0 Comments